What Is Diabetic Ketoacidosis
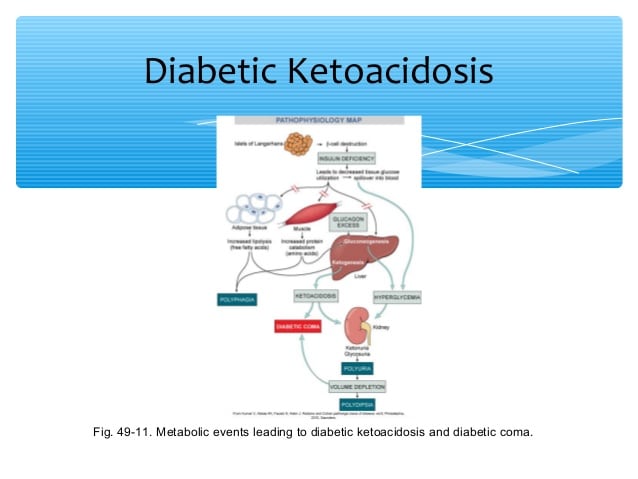
Diabetic ketoacidosis is a condition that can affect people with diabetes, usually those with type 1 diabetes. People with type 2 diabetesor diabetes in pregnancy can also have diabetic ketoacidosis but this is much rarer. Diabetic ketoacidosis happens when your body does not have enough insulin to help it use sugar for energy. Instead, your body starts burning fat for energy, which releases harmful ketones. A build-up of ketones in your body causes your blood to become acidic. This is why it is called ketoacidosis.
Because diabetic ketoacidosis upsets the chemical balance in your body, it can quickly become dangerous. It needs immediate medical attention. It is isn’t treated, diabetic ketoacidosis can lead to coma and even death. If picked up early, it can be treated with extra insulin, glucose and fluid.
Why Is Hyperkalemia A Cause Of Concern
Hyperkalemia can have far-reaching effects on our body. It has several adverse health effects which are why you should be wary of this condition.
While potassium is necessary for the effective functioning of the heart and different muscles of the body, excessive levels of potassium should be avoided at the same time.
- Hyperkalemia can cause interruptions in the electrical activity of the heart and can ultimately cause the heart to stop functioning.
- Muscle paralysis can also be caused due to high levels of potassium in the body
- Finally, the condition has far-reaching adverse effects on the skeletal muscles
We hope that the above article has helped you to understand more about hyperkalemia and how diabetic ketoacidosis can cause the condition. It can be really severe and as such, you should be able to effectively manage your diabetes and diabetic ketoacidosis so that you can avoid suffering from the high levels of potassium in your blood!!
The Normal Range Of Potassium In The Body
Potassium has the capacity to move out of and into the cells of the body. Total stores of potassium in the body are 50 mEq/kg of body weight. Out of this, about 98% of the total potassium is located inside the body cells and is intracellular. Only 2% is located outside the cells in blood circulation and in extracellular tissues.
Blood tests only measure potassium levels that are outside the cells, in the blood circulation. So, conditions and diseases that cause potassium to move out of the cells into the blood stream can increase the levels of potassium into the blood, even though the total amount of potassium has not changed in the body.
Potassium is a mineral that helps to keep the body fluids at a normal level.So, by keeping fluids in check it helps muscles to contract without pain, keeps the heart beating correctly, and maintains brain function at its highest capacity.
Read Also: How Many Points Does Metformin Lower Blood Sugar
Quality Of The Included Studies
In terms of quality of the studies, 22 studies31â39,41,42,44,46,49,52â54,56,58,59,61,71 scored poor, 18 studies9,16,21,45,47,48,50,51,60,62,63,66,68â70,72â74 scored fair, and two studies64,65 scored good on NOS. Among studies scoring 5, four 43,55,62,72 were scored on the basis of comparability by controlling confounding factors between the groups as well. Similarly, of the studies that scored 3 and 4 on NOS, three studies33,58,61 from score 3, and two studies47,57 from score 4 were also scored for controlling confounding factors between the groups . Of four studies that established comparability between respondents and non-respondents, one each scored 440 and 657 on NOS and two scored 7.64,67 Notably, there was only one study that defined the sample size in terms of calculating on the basis of prevalence of DKA.69
The Cochrane Risk of Bias tool was used to assess five studies.40,43,55,57,67 None of the studies was completely blinded for participants . In the context of blinding of outcomes, only two of the studies55,67 predefined outcomes for assessment of endpoints. Among these studies, the least reporting bias was observed in the study by Ersöz et al,55 while the rest of the studies were found with a moderate risk of bias.40,43,57,67
How Is Hyperkalemia Treated
Treatment of hyperkalemia must be individualized based upon the underlying cause of the hyperkalemia, the severity of symptoms or appearance of ECG changes, and the overall health status of the patient. Mild hyperkalemia is usually treated without hospitalization especially if the patient is otherwise healthy, the ECG is normal, and there are no other associated conditions such as acidosis and worsening kidney function. Emergency treatment is necessary if hyperkalemia is severe and has caused changes in the ECG. Severe hyperkalemia is best treated in the hospital, oftentimes in the intensive care unit, under continuous heart rhythm monitoring.
Treatment of hyperkalemia may include any of the following measures, either singly or in combination:
Treatment of hyperkalemia also includes treatment of any underlying causes of hyperkalemia.
You May Like: Articles About Diabetes Mellitus
Identification And Treatment Of Underlying Causes And Prevention Of Recurrence Of Diabetic Ketoacidosis Or Hyperosmolar Hyperglycemic Syndrome
Assess and manage reasons for insulin non-adherence
Consider alternative insulin regimens and/or social work consultation if the patient is unable to afford the prescribed insulin
Facilitate treatment or social services for patients with untreated or poorly controlled psychiatric disorders
Refer patients with substance or alcohol use disorders to treatment programs
Provide education on appropriate sick day management and refer the patient to a formal diabetes education program after hospital discharge
Treat treatable precipitating causes such as infection, cerebrovascular accident, myocardial infarction, or trauma
All patients with diabetes should receive initial and ongoing education about diabetes in the community setting, as this can help to prevent diabetes related hospital admissions . This education is best provided or directed by a trained diabetes educator.868990 Patient specific instructions for sick day management, including reminders that intermediate or long acting insulin should never be stopped and when to call for assistance, can help to prevent future episodes of diabetic ketoacidosis.9192 All patients with type 1 diabetes and many insulin treated patients with type 2 diabetes should have a means to measure ketones at home and be instructed to monitor both glucose and ketones at least every four hours during illness.
Clinical Presentation And Diagnosis
Table 1 and table 2 outline diagnostic criteria for diabetic ketoacidosis and HHS as recommended by the American Diabetes Association , Joint British Diabetes Societies for Inpatient Care, and American Association of Clinical Endocrinologists. Diagnostic criteria for diabetic ketoacidosis can include an elevation of urine acetoacetate or blood β-hydroxybutyrate. Point of care blood ketone meters and test strips for measurement of β-hydroxybutyrate are costly and not readily available in many institutions, but they are likely to become standard of care over time as they also provide accurate information for guiding treatment .36
You May Like: Can You Be Born With Type 2 Diabetes
Evolution And Species Distribution
Insulin may have originated more than a billion years ago. The molecular origins of insulin go at least as far back as the simplest unicellular . Apart from animals, insulin-like proteins are also known to exist in the Fungi and Protista kingdoms.
Insulin is produced by of the in most vertebrates and by the in some . and , venomous sea snails that hunt small fish, use modified forms of insulin in their venom cocktails. The insulin toxin, closer in structure to fishes’ than to snails’ native insulin, slows down the prey fishes by lowering their blood glucose levels.
Evaluating The Cause Of Dka
precipitating cause
DKA is occasionally the initial manifestation of diabetes, but it usually occurs in the context of known diabetes plus a trigger. This is especially true of patients with type-II DM, who don’t generally require exogenous insulin but may develop DKA in the context of physiologic stress. Most triggers of DKA are benign . However, DKA can be caused by any source of physiologic stress. Occasionally, DKA is the presentation of a serious underlying problem, especially sepsis. Common triggers of DKA include:
- Absolute insulin deficiency:
evaluation for the cause of DKA
- History and physical examination are the key here. If there is clear history of nonadherence, a big workup isn’t necessary.
- Infectious trigger?
- DKA itself may cause leukocytosis, so a WBC elevation alone is nonspecific.
- Infection is suggested by fever, marked left-shift, or severe leukocytosis .
Don’t Miss: High Blood Sugars Side Effects
Treatment Of Dka In Dialysis Patients
Diabetes is a leading cause of end-stage kidney disease in the US. DKA is infrequent in dialysis patients but it has been increasingly encountered due to rising prevalence of diabetic ESKD. The kidney plays an important role in glucose homeostasis, and the loss of kidney function is often associated with improved glycemic control due to the reduction of kidney gluconeogenesis, improved insulin sensitivity with regular dialysis, and reduced insulin clearance. However, these processes also place ESKD patients with diabetes at higher risk of hypoglycemia.
It is important to note that no prospective studies have systematically evaluated strategies assessing treatment and resolution of DKA in dialysis patients therefore, the diagnosis of DKA and monitoring for its resolution should be done in close collaboration with a nephrologist. Insulin administration is a mainstay and frequently the only treatment required for DKA management in dialysis patients. In the absence of data from prospective studies, the initial rate of intravenous insulin administration for dialysis patients should be similar to non-dialysis individuals, with a recommended pace of serum glucose decline of 100125 mg/dL/h to avoid neurologic consequences from a rapid reduction in serum tonicity and intracellular swelling. In our opinion, initial insulin bolus to 0.1 U/kg followed by continuous insulin infusion at 0.05 U/kg/h may be appropriate to avoid too rapid glucose correction.
How Is Hyperkalemia Diagnosed
Blood is withdrawn from a vein . The potassium concentration of the blood is determined in the laboratory. If hyperkalemia is suspected, an electrocardiogram is often performed, since the ECG may show changes typical for hyperkalemia in moderate to severe cases. The ECG will also be able to identify cardiac arrhythmias that result from hyperkalemia.
You May Like: Does Crystal Light Raise Blood Sugar
Recommendations For Potassium Resuscitation
As mentioned earlier, late-sought medical attention for DKA causes the treatment to be complex as well. Under normal conditions, i.e., when patient is not comatose, the patient is mostly started on either intravenous or basal bolus insulin, and normal saline. As per recommendation of various guidelines, the important biochemical profiles include analysis of blood gasses and renal profile. It is widely suggested that the normal saline shall be used for initial resuscitation and once the potassium level is retrieved, the patient can be started on potassium replacement should the serum potassium level be between 3.3 and 4.5 mmol/L . All the major guidelines further acknowledge that insulin mediated reentry of potassium from extracellular to intracellular compartment may precipitate hypokalemia, and hence, insulin should be withheld if the serum level of potassium is below 3.3 mmol/L. Although that the potassium level is given appropriate representation , however, the extent of being deceived with the retrieved level of potassium may end up in a lethal outcome.
Table 1. Recommendation of potassium replacement by different guidelines.
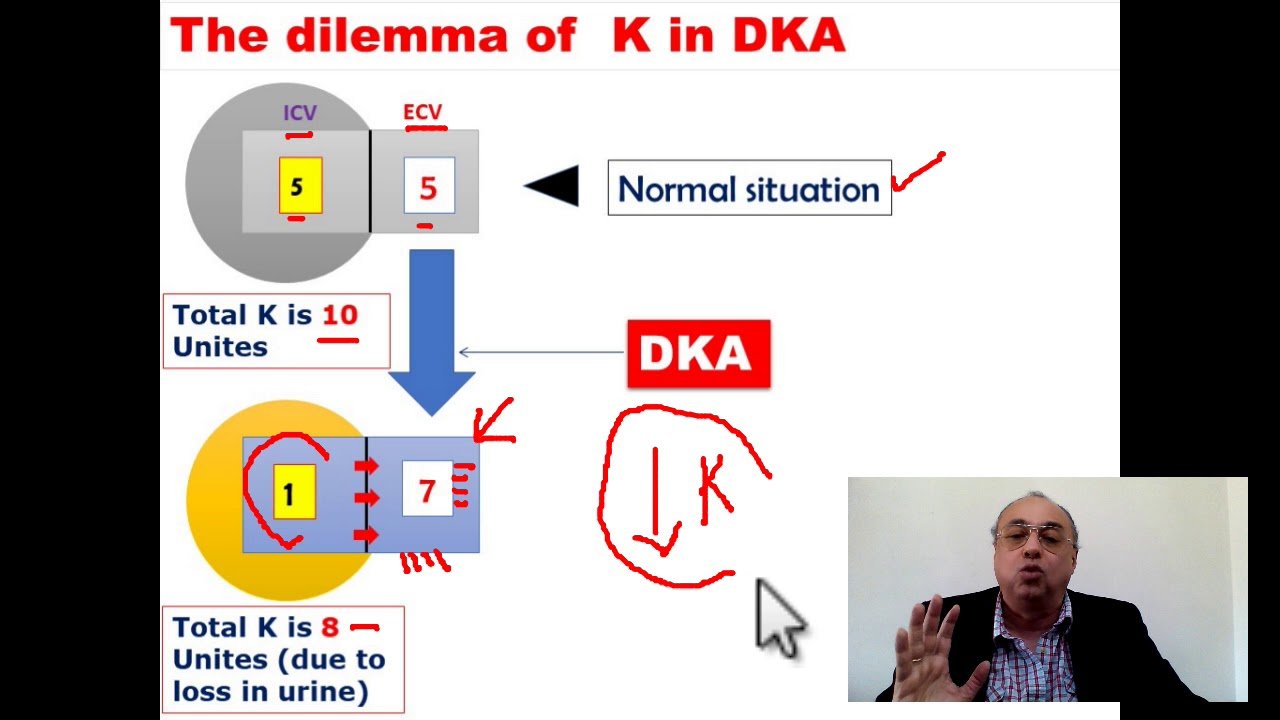
Relationship Of Insulin To Potassium
Insulin is the hormone that is secreted by the beta cells of the pancreas. It keeps the level of potassium within the normal range in the blood. When insulin decreases the potassium level increases.
Insulin has plenty of functionsin the body besides lowering blood sugar. One of its functions is to put potassium into the cells by activating the cellular channel of sodium and potassium. Insulin stimulates the uptake of potassium and glucose in all the bodys cells, but primarily it fuels the fat cells as well as muscle cells.
In type 2 diabetes insulin does not function properly. Therefore, the body cells become resistant to insulin and blood glucose levels are elevated.
You May Like: Are You Born With Type 2 Diabetes
Relation Of Low Potassium And Risk Of Diabetes
It is believed that potassium causes the release of insulin from the beta cells of the pancreas. Low levels of potassium can contribute to the stress of the pancreas and result in future insulin resistance, leading to type 2 diabetes mellitus. It has been noticed that a higher intake of dietary potassium may result in a reduced risk of diabetes mellitus.
Clinical Approach To Anion Gap & Ketoacidosis
- Obvious DKA: In some cases the history and physical examination are strongly suggestive of DKA. In this situation, an elevated anion gap with positive urinary ketones may be sufficient to reach a diagnosis of DKA.
- Complicated cases: In confusing situations, it’s helpful to simultaneously measure electrolytes, lactate, and a beta-hydroxybutyrate level. This can help sort out the precise etiology of the elevated anion gap, for example:
- A strongly elevated beta-hydroxybutyrate level would support a diagnosis of DKA.
- A markedly elevated lactate level with mildly elevated beta-hydroxybutyrate level might suggest an underlying disease process that may be causing a mild amount of ketoacidosis.
- An elevated anion gap with normal lactate and beta-hydroxybutyrate levels implies an alternative cause of the anion gap .
Recommended Reading: How Long Does Someone With Diabetes Live
Three Ways To Evaluate For Ketoacidosis
anion gap
- Anion Gap =
- Using this formula, an elevated anion gap is above 10-12 mEq/L.
- Please don’t correct for albumin, glucose, or potassium. Don’t make this unnecessarily complicated.
urinary dipstick for ketones
- The urinary ketone dipstick tests for acetoacetate.
- This test has a high sensitivity for DKA , with urinary ketones are generally being 2+. False negatives may occur in patients with highly acidic urine.
- The specificity of a positive measurement of urinary ketones is low, so a positive urinary measurement of ketones doesn’t establish a diagnosis of DKA. For example, starvation ketoacidosis is a more common cause of urinary ketones in most contexts.
blood beta-hydroxybutyrate level
- > 3 mM: Consistent with DKA.
- > 6 mM: Severe DKA.
Regulator Of Endocannabinoid Metabolism
Insulin is a major regulator of and insulin treatment has been shown to reduce ECs, the and , which correspond with insulin-sensitive expression changes in enzymes of EC metabolism. In insulin-resistant , patterns of insulin-induced enzyme expression is disturbed in a manner consistent with elevated EC and reduced EC degradation. Findings suggest that adipocytes fail to regulate EC metabolism and decrease intracellular EC levels in response to insulin stimulation, whereby insulin-resistant individuals exhibit increased concentrations of ECs. This dysregulation contributes to excessive accumulation and reduced release from abdominal adipose tissue, and further to the onset of several cardiometabolic risk factors that are associated with obesity and .
, also known as “low blood sugar”, is when decreases to below normal levels. This may result in a variety of including clumsiness, trouble talking, confusion, , or death. A feeling of hunger, sweating, shakiness and weakness may also be present. Symptoms typically come on quickly.
The most common cause of hypoglycemia is used to treat such as insulin and . Risk is greater in diabetics who have eaten less than usual, exercised more than usual or have drunk . Other causes of hypoglycemia include , certain , such as , , , , , , and a number of drugs including alcohol. Low blood sugar may occur in otherwise healthy babies who have not eaten for a few hours.
Recommended Reading: 1500 Mg Metformin
Pseudomyocardial Infarction In A Patient With Severe Diabetic Ketoacidosis And Mild Hyperkalemia
Edgar Francisco Carrizales-Sepúlveda
1Internal Medicine Department, Hospital Universitario, Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, Mexico
2Echocardiography Laboratory, Cardiology Service, Hospital Universitario, Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, Mexico
Abstract
1. Introduction
Heart tissue is particularly prone to the effects of systemic acidosis and hyperkalemia . Acidosis decreases myocardial contractibility by affecting the excitation-contraction coupling , and hyperkalemia causes depolarization of the cardiac-cell resting membrane potential, shortening of the action potential duration, and alterations in the conduction velocity . Diabetic ketoacidosis is considered one of the most serious acute complications of diabetes mellitus it is characterized by hyperglycemia, metabolic acidosis, and increased total body ketone concentrations . Despite volume depletion seen in DKA secondary to vomiting and reduced oral intake, serum potassium levels are typically high at presentation this is because lack of insulin and the presence of acidosis cause a shift of potassium from the intracellular space to extracellular space which usually resolve with DKA treatment . Thus, as a consequence of the acid-base and potassium derangements, patients with DKA can present electrocardiographic alterations than can be transient and resolve with treatment.
2. Case Report
3. Discussion
4. Conclusion
Conflicts of Interest
Intubating A Dka Patient
- Whenever possible, avoid intubation.
- Intubating a patient due to altered mental status is usually a mistake. The mental status should improve over several hours, so careful observation is generally the best approach.
- Indications for intubation may include:
- Frank inability to protect airway .
- Intubation needed to facilitate surgical procedure .
- Respiratory arrest or impending arrest .
risks involved with intubation
mitigating the risks
- Volume resuscitate prior to intubation.
- If necessary start a vasopressor infusion to establish MAP > 75-80mm before the procedure.
- Use hemodynamically stable induction drugs .
Recommended Reading: Which Pancreatic Cells Release Insulin And Glucagon

