Glucagon And Insulin Secretion Is Inhibited By Exogenous And Endogenous Somatostatin
Initially, we confirmed that 0.1 nmol/l somatostatin infusion powerfully inhibits secretion of glucagon and insulin in the perfused mouse pancreases. This inhibitory effect on insulin and glucagon was further demonstrated with infusion of somatostatin receptor antagonists. To obtain full blockade of the somatostatin receptor subtypes expressed in the pancreatic islets, we used a cocktail of somatostatin receptor antagonists PRL2915 and PRL3195, which are inhibitors of somatostatin receptor 2, SSTR3 and SSTR5 . This combination successfully blocked any effect of exogenous somatostatin on insulin and glucagon secretion and was used in the subsequent experiments. Glucagon secretion increased ~1.8-fold at 3.5 mmol/l glucose, and?~?4.5-fold at 12 mmol/l glucose, reflecting higher glucose-stimulated somatostatin levels. Somatostatin receptor blockade had no effect on insulin secretion at low glucose, but significantly increased insulin secretion at 12 mmol/l glucose . Together, these experiments confirmed the strong tonic inhibitory role of somatostatin on glucagon and insulin secretion as previously demonstrated . Importantly, somatostatin does not seem to have an inhibitory role on insulin secretion at low glucose, but only on glucagon secretion.
Fig. 1Full size image
How Has Defining The Role Of Gpcrs In Islet Function Translated Into Drug Discovery
This topic has been reviewed in detail by Ahren and colleagues , but one example is the success that has been achieved in the treatment of type 2 diabetes by targeting the glucagon like peptide-1 receptor , which is a GPCR. In the 1970s and 80s, scientists defined a new set of molecules, incretins, which stimulate glucose-dependent insulin secretion when released by cells in the gut following a meal. Incretins include GLP-1 and glucose-dependent insulinotropic polypeptide . When secreted by cells in the gastrointestinal tract, GLP-1 acts through its receptor on pancreatic islet cells to stimulate insulin secretion and inhibit glucagon secretion. Knowing that, targeting the GLP-1 receptor has been a major initiative in drug development research. New drugs capable of binding to the GLP-1R are now approved as a treatment for type 2 diabetes. These drugs not only enhance insulin secretion and inhibit glucagon secretion, but clinical researchers also noted that treatment with a GLP-1R agonist decreased appetite and induced early satiety, which resulted in significant weight loss in many patients. It is speculated that the effect on appetite is mediated through receptors in the brain.
Defect In Pancreas Alpha Cells Linked To Diabetes Stanford Medicine Study Shows
Pancreatic alpha cells from people with diabetes release excess amounts of glucagon, a hormone important in blood sugar control, in a new Stanford-developed mouse model of transplanted human islets.
Research by Seung Kim and others at Stanford Medicine suggests that diabetes stems from defects in more than one type of cells.Steve Fisch
In response to low blood sugar levels, pancreatic islet cells from people with diabetes release significantly more of a hormone called glucagon than do islet cells from healthy people, according to a new study by researchers at the Stanford University School of Medicine.
The discovery was made using a new mouse model of diabetes engineered by Stanford scientists that for the first time permits functional studies of transplanted human alpha cells.
Glucagon is produced by alpha cells in pancreatic islets while insulin is produced by beta cells. Defects of insulin output and beta cells have been thought to be the main drivers of diabetes. The current study, however, supports the growing realization that diabetes is likely due to defects in multiple cell types and highlights the importance of the mouse model to more accurately simulate the complexities of the disease.
Kim is the senior author of the study, which was published June 8 in Nature Metabolism. Graduate student Krissie Téllez and research scientist Yan Hang, PhD, share lead authorship of the study.
Which Type Of Diabetes Is Caused By The Pancreas Not Producing Enough Insulin
Type 1 diabetes happens when the pancreas does not make enough, or any, insulin. Without insulin, the cells cannot get enough energy from food. This form of diabetes results from the body’s immune system attacking the insulin-producing beta cells in the pancreas.
READ: What can kill a newborn puppy?
The Important Roles Of Insulin And Glucagon: Diabetes And Hypoglycemia
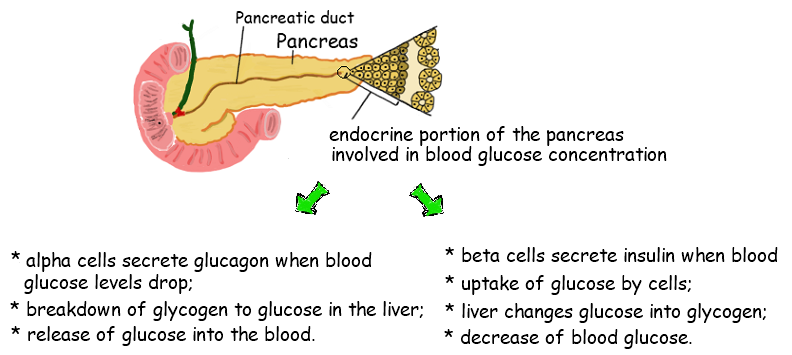
The human body wants blood glucose maintained in a very narrow range. Insulin and glucagon are the hormones which make this happen. Both insulin and glucagon are secreted from the pancreas, and thus are referred to as pancreatic endocrine hormones. The picture on the left shows the intimate relationship both insulin and glucagon have to each other. Note that the pancreas serves as the central player in this scheme. It is the production of insulin and glucagon by the pancreas which ultimately determines if a patient has diabetes, hypoglycemia, or some other sugar problem.
Insulin Basics: How Insulin Helps Control Blood Glucose Levels
Insulin and glucagon are hormones secreted by islet cells within the pancreas. They are both secreted in response to blood sugar levels, but in opposite fashion!
Insulin is normally secreted by the beta cells of the pancreas. The stimulus for insulin secretion is a HIGH blood glucose…it’s as simple as that! Although there is always a low level of insulin secreted by the pancreas, the amount secreted into the blood increases as the blood glucose rises. Similarly, as blood glucose falls, the amount of insulin secreted by the pancreatic islets goes down.
As can be seen in the picture, insulin has an effect on a number of cells, including muscle, red blood cells, and fat cells. In response to insulin, these cells absorb glucose out of the blood, having the net effect of lowering the high blood glucose levels into the normal range.
Glucagon is secreted by the alpha cells of the pancreatic islets in much the same manner as insulin…except in the opposite direction. If blood glucose is high, then no glucagon is secreted.
When blood glucose goes LOW, however, more and more glucagon is secreted. Like insulin, glucagon has an effect on many cells of the body, but most notably the liver.
Modulation Of Glucagon Action And Glucagon Receptor Signalling
Peptide-based glucagon receptor antagonists
Several linear and cyclic glucagon analogues have been developed to work as glucagon receptor antagonists. Essentially, they impair the ability of glucagon to stimulate adenylate cyclase activity in liver, thus reducing hepatic glucose output and improving plasma glucose levels. This is the case of glucagon-NH2, which reduces glucose levels in streptozotocin-induced diabetic rats . Recent investigations have demonstrated that the antagonist des-His-glucagon binds preferentially to the hepatic glucagon receptor in vivo, and this correlates with the glucose lowering effects .
Non-peptide glucagon receptor antagonists
Multiple, competitive and non-competitive, non-peptide antagonists have been reported to act on glucagon binding and/or function. For instance, a novel competitive antagonist was recently shown to inhibit glucagon-mediated glycogenolysis in primary human hepatocytes and to block the increase in glucose levels after the administration of exogenous glucagon in mice . The information about the effect of these antagonists on humans is, however, scarce. In this respect, Bay 27-9955 is an oral glucagon receptor antagonist that has been tested in humans, demonstrating its efficacy in reducing glucose levels induced by exogenous glucagon .
The Precious Pancreas: Insulin Glucagon And Digestive Juices
Posted on 10/10/19 by Adelaide Elkin
Located behind the stomach and at the back of the abdomen is the six-inch-long, tadpole-shaped pancreas. From head to tail, the pancreas extends across the abdomen. The head is based where the stomach meets the duodenum .
The pancreas in context. Image from Human Anatomy Atlas.
The pancreas is an accessory digestive organ made of small glandular clusters of epithelial cells. It’s crucial for converting food to fuel for body cells and contains both endocrine and exocrine gland cells.
Regulation Of Blood Glucose Levels By Insulin And Glucagon
Glucose is required for cellular respiration and is the preferred fuel for all body cells. The body derives glucose from the breakdown of the carbohydrate-containing foods and drinks we consume. Glucose not immediately taken up by cells for fuel can be stored by the liver and muscles as glycogen, or converted to triglycerides and stored in the adipose tissue. Hormones regulate both the storage and the utilization of glucose as required. Receptors located in the pancreas sense blood glucose levels, and subsequently the pancreatic cells secrete glucagon or insulin to maintain normal levels.
Diabetes Mellitus Causes Increased Utilization Of Fats And
keto acids into the plasma more rapidly than they can be taken up and oxidized by the tissue cells. As a result, the patient develops severe metabolic acidosis from the excess keto acids. This leads rapidly to diabetic coma and death unless the condition is treated immediately with large amounts of insulinDiabetes Causes Depletion of the Body’s Proteins? a patient with severe untreated diabetes mellitus suffers rapid
Vcac: Cellular Processes: Insulin Signaling: First Look
Insulin Signaling: First Look The following images attempt to illustrate the major events involved in the insulin signaling pathway responsible for increasing a cell’s glucose uptake. Clicking on each of the thumbnail images will bring up a larger, labeled version of the described scene. To see the Flash movie for the following sequence of images, click here . When blood glucose levels rise, insulin from the pancreas travels through the blood stream to a fat cell. Insulin then binds to an Insulin Receptor found in the cell’s plasma membrane. Phosphate groups are then added to the IR through the process of autophosphorylation. Several additional proteins are in turn phosphorylated as the signal is transferred down the pathway. One of the last proteins in the pathway then moves away from the plasma membrane towards the GLUT4 storage vesicle pool. When the insulin signal pathway is inactive, GLUT4 storage vesicles are held in a recycling state, a short distance from the cell membrane. When the signal that was originally sent by insulin reaches the GSV pool, the vesicles are released from their holding pattern and move toward the plasma membrane. The GSV pool then merges with the plasma membrane allowing many additional GLUT4 proteins to transport glucose into the cell. This leads to a a great increase in the amount of glucose taken in by the target cells.Continue reading >>
Insulin And Glucagon Influences On Glucose Homeostasis
Ktorza and associates12 evaluated insulin and glucagon secretion during the perinatal period, and noted that insulin and glucagon are detected in most species early in gestation. The insulin-glucagon molar ratio is high in the fetus at term, but then decreases after birth and remains low during the first hours after birth. This change favors glycogenolysis and gluconeogenesis after birth. King and colleagues13 studied postnatal development of insulin secretion in the premature neonate for 110 days after birth and in the term neonate for up to 47 days after birth. Insulin levels were measured before and 30 minutes after administration of glucose parenterally or enterally. Premature neonates exhibited a small increase in insulin secretion in response to glucose administration on postnatal day 1, which gradually increased over the course of the study. The term neonate secreted more insulin than preterm infants. The investigators concluded that the premature neonate may take up to 18 weeks to develop a fully mature response to an increase in plasma glucose concentration.
Glucagon Control Of Glucose Homeostasis And Metabolism
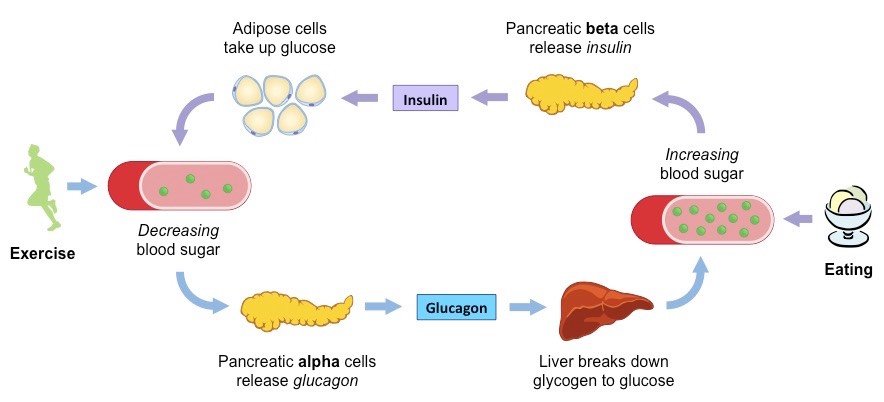
Glucagon can also stimulate the uptake of amino acids for gluconeogenesis in the liver. Indeed, subjects with hyperglucagonaemia can develop plasma hypoaminoacidaemia, especially of amino acids involved in gluconeogenesis, such as alanine, glycine and proline . Glucagon is also involved in the regulation of fatty acids in adipocytes. Hormone-sensitive lipase mediates the lipolysis of triacylglycerol into the non-esterified fatty acids and glycerol, which are released from adipocytes. It has been reported that although glucagon does not modify the transcriptional levels of this enzyme, it increases the release of glycerol from adipocytes . This mobilization of glycerol from adipose tissue can further be used in the liver during gluconeogenesis. However, the existence of a lipolytic action of glucagon observed in several animal models is still controversial in humans. While a positive effect of glucagon on lipolysis has been reported in human subjects , several recent studies have indicated that it lacks a role in a physiological context . An elevated glucagon to insulin ratio accelerates gluconeogenesis as well as fatty acid ?-oxidation and ketone bodies formation . Thus, glucagon may also be involved in diabetic ketoacidosis, a medical complication in diabetes derived from the overproduction of ketone bodies .
What Is The Relationship Insulin Glucose And The Cell
Glucose, a simple sugar, provides energy for cell functions. After food is digested, glucose is released into the bloodstream. In response, the pancreas secretes insulin, which directs the muscle and fat cells to take in glucose. Cells obtain energy from glucose or convert it to fat for long-term storage.
READ: How do you get rid of valve train noise?
How Do You Know If Your Pancreas Is Producing Insulin
C-peptide and insulin are released from the pancreas at the same time and in about equal amounts. So a C-peptide test can show how much insulin your body is making. This test can be a good way to measure insulin levels because C-peptide tends to stay in the body longer than insulin.
READ: Is a can opener a compound machine?
Production Of Hormones To Control Blood Sugar Levels
A small proportion of the pancreas is made up of other types of cells called islets of Langerhans. These cells sit in tiny groups, like small islands, scattered throughout the tissue of the pancreas. The islets of Langerhans contain alpha cells which secrete glucagon and beta cells which secrete insulin.
Insulin and glucagon are hormones that work to regulate the level of sugar in the body to keep it within a healthy range. Unlike the acinar cells, the islets of Langerhans do not have ducts and secrete insulin and glucagon directly into the bloodstream.
Depending on what you’ve eaten, how much exercise your muscles are doing, and how active your body cells are, the amount of glucose in your bloodstream and cells varies. These 2 hormones have the job of keeping tight control of the amount of glucose in your blood so that it doesn’t rise or fall outside of healthy limits.
Which Pancreatic Cells Release Insulin And Glucagon
4.4/5isletbeta cells
Insulin is released by the ‘beta cells‘ in the islets of Langerhans in response to food. Its role is to lower glucose levels in the bloodstream and promote the storage of glucose in fat, muscle, liver and other body tissues. ‘Alpha cells‘ in the islets of Langerhans produce another important hormone, glucagon.
Likewise, how is the secretion of insulin and glucagon regulated? Insulin helps the cells absorb glucose, reducing blood sugar and providing the cells with glucose for energy. When blood sugar levels are too low, the pancreas releases glucagon. Glucagon instructs the liver to release stored glucose, which causes blood sugar to rise.
Similarly, which pancreatic cells release insulin and glucagon quizlet?
Liver cells, as well as most other cells of the body. The pancreas releases glucagon, which eventually causes blood glucose levels to increase.
What stops the pancreas from producing insulin?
Researchers have discovered that patients with type 1 diabetes can regain the ability to produce insulin. The disease causes the pancreas to stop producing insulin, a hormone that regulates blood sugar levels. When blood sugar levels are too high, the smallest blood vessels in the body eventually become damaged.
Here are 14 natural, science-backed ways to boost your insulin sensitivity.
Glucagon May Regulate Heart Rate And Contractility
Infusion of high doses of glucagon increases heart rate and cardiac contractility . In fact, infusion of glucagon in pharmacological doses is often used in the treatment of acute cardiac depression caused by calcium channel antagonist or beta-blocker overdoses despite limited evidence . In comparison, glucagon concentrations within the normal physiological range do not appear to affect heart rate or contractility and any physiological role of endogenous glucagon in the regulation of pulse rate remains questionable. This is supported by studies investigating the effect of glucagon receptor antagonist for the treatment of type 2 diabetes in which no effect of pulse rate were observed . Nevertheless, whether increased glucagon concentrations have a sustained effect on the heart remains unknow. Of note, most studies use bolus injections of glucagon which cause only a transient increase in heart rate and contractility . Taken together, it remains uncertain whether glucagon has a place in the treatment of heart failure or hold a cardioprotective effect in healthy subjects.
Endocrine And Exocrine Functions Of The Pancreas
The exocrine cells of the pancreas produce enzymes that combine with electrolytes and water to become part of a digestive fluid called pancreatic juice. It is carried by a network of pancreatic ducts to the small intestine. The juice promotes digestion as it helps break down carbs, fats, and proteins. About 1200-1500 mL of pancreatic juice is produced daily!
The pancreas , its ducts, and the duodenum. Image from Human Anatomy Atlas.
The pancreas also contains around a million pancreatic islets , which are hormone-producing cell clusters. The islets contain two major populations of hormone producing cells: the glucagon-synthesizing alpha cells and the insulin-synthesizing beta cells. These cells secrete glucagon or insulin during the fasting and full states and can sense when the body needs and doesn’t need food.
Cells And Secretions Of The Pancreatic Islets
The pancreatic islets each contain four varieties of cells:
- The alpha cell produces the hormone glucagon and makes up approximately 20 percent of each islet. Glucagon plays an important role in blood glucose regulation; low blood glucose levels stimulate its release.
- The beta cell produces the hormone insulin and makes up approximately 75 percent of each islet. Elevated blood glucose levels stimulate the release of insulin.
- The delta cell accounts for four percent of the islet cells and secretes the peptide hormone somatostatin. Recall that somatostatin is also released by the hypothalamus , and the stomach and intestines also secrete it. An inhibiting hormone, pancreatic somatostatin inhibits the release of both glucagon and insulin.
- The PP cell accounts for about one percent of islet cells and secretes the pancreatic polypeptide hormone. It is thought to play a role in appetite, as well as in the regulation of pancreatic exocrine and endocrine secretions. Pancreatic polypeptide released following a meal may reduce further food consumption; however, it is also released in response to fasting.
The Role Of Glucagon In Blood Glucose Control
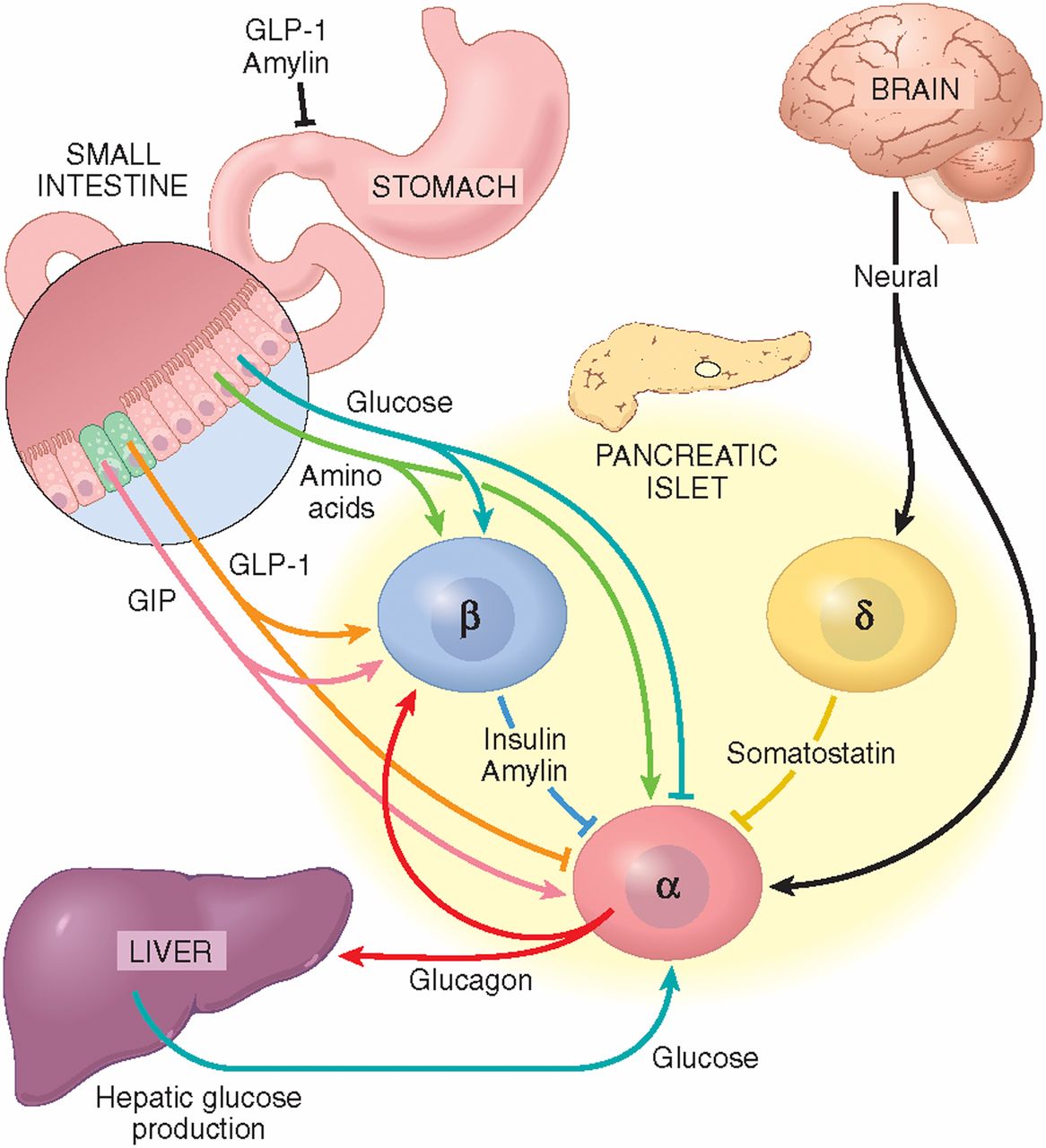
The effect of glucagon is to make the liver release the glucose it has stored in its cells into the bloodstream, with the net effect of increasing blood glucose. Glucagon also induces the liver to make glucose out of building blocks obtained from other nutrients found in the body .
Our bodies desire blood glucose to be maintained between 70 mg/dl and 110 mg/dl . Below 70 is termed “hypoglycemia.” Above 110 can be normal if you have eaten within 2 to 3 hours. That is why your doctor wants to measure your blood glucose while you are fasting…it should be between 70 and 110. Even after you have eaten, however, your glucose should be below 180. Above 180 is termed “hyperglycemia” . If your 2 two blood sugar measurements above 200 after drinking a sugar-water drink , then you are diagnosed with diabetes.
Glucagon Increases Hepatic Glucose Production
Glucagon controls plasma glucose concentrations during fasting, exercise and hypoglycemia by increasing hepatic glucose output to the circulation. Specifically, glucagon promotes hepatic conversion of glycogen to glucose , stimulates de novo glucose synthesis , and inhibits glucose breakdown and glycogen formation . Hepatic glucose production is rapidly enhanced in response to a physiological rise in glucagon; achieved through stimulation of glycogenolysis with minor acute changes in gluconeogenesis . This ability of glucagon is critical in the life-saving counterregulatory response to severe hypoglycemia. Additionally, it is a key factor in providing adequate circulating glucose for brain function and for working muscle during exercise . During prolonged fasting, glycogen stores are depleted, and gluconeogenesis takes over . The hyperglycemic property of glucagon is enhanced when hepatic glycogen levels are high and diminished when hepatic glycogen levels are low in conditions of fasting or liver diseases like cirrhosis .
Somatostatin Secretion Requires Normal Intra
To investigate the intra-islet paracrine effects of endogenous glucagon on somatostatin secretion, we used transgenic mouse models to disrupt glucagon signalling. We have previously utilised DT-induced destruction of proglucagon cells in transgenic mice expressing the human DT receptor under control of the proglucagon promoter to acutely knock down the alpha cells and thereby glucagon secretion. These mice also exhibited significantly reduced insulin secretion, indicating that normal glucagon secretion is necessary to maintain appropriate insulin secretion . We here report that somatostatin secretion in these mice was likewise reduced compared with control mice. They were still able to secrete somatostatin in response to GLP-1 showing that the delta cells are still functional although with a lower level of basal somatostatin secretion. The lack of an arginine-stimulated response may therefore reflect a need for a rise in glucagon for arginine-stimulated somatostatin secretion, whereas GLP-1 may directly stimulate somatostatin through GLP-1 receptors on the delta cells. However, it is also possible that a basic tone of glucagon stimulation is required to maintain normal somatostatin secretion, without which responses to other stimuli are lost.
Regulation Of Glucagon Secretion By Glucose
The most potent regulator of glucagon secretion is circulating glucose. Hypoglycemia stimulates the pancreatic alpha cell to release glucagon and hyperglycemia inhibits glucagon secretion . The cellular mechanism behind this glucose-dependent regulation of glucagon secretion involves uptake of glucose by the glucose transporter 1 in the cell membrane of pancreatic alpha cells and subsequent glycolysis which ultimately generates adenosine triphosphate in the mitochondria of the alpha cell. Thus, the intracellular ATP level in the alpha cell reflects plasma glucose levels. Hypoglycemia and resulting low intracellular ATP levels in the alpha cell close ATP-sensitive potassium channels whereby the efflux of potassium is reduced. This causes a depolarization of the cell membrane which, in turn, opens voltage-dependent Ca2+ channels allowing influx of Ca2+. This increases intracellular Ca2+ levels, the primary trigger for exocytosis of glucagon granules from the alpha cells . Conversely, increasing circulating glucose levels increase glucose influx to the alpha cell generating an increase in intracellular ATP concentration, which opens KATP-channels. This leads to a membrane potential that closes voltage-dependent Ca2+ channels thereby preventing Ca2+ influx and glucagon secretion .
What Happens If I Have Too Little Glucagon
Unusual cases of deficiency of glucagon secretion have been reported in babies. This results in severely low blood glucose which cannot be controlled without administering glucagon.
Glucagon can be given by injection to restore blood glucose lowered by insulin . It can increase glucose release from glycogen stores more than insulin can suppress it. The effect of glucagon is limited, so it is very important to eat a carbohydrate meal once the person has recovered enough to eat safely.
Glucagon Concentrations In The Circulation
In normal physiology, circulating glucagon concentrations are in the picomolar range. In the fasting state with plasma glucose levels around 5 mmol/l, glucagon is secreted in basal levels resulting in plasma concentrations below 20 pmol/l . Basal glucagon secretion balances the effect of basal insulin secretion resulting in a steady-state between glucose uptake and endogenous glucose production in the fasted state; i.e. stable blood glucose concentrations. During exercise or in case of hypoglycemia, circulating glucagon levels may increase dramatically to 3-4 times basal levels increasing the glucagon to insulin ratio .
Gpcrs And Insulin Changes During Pregnancy
A variety of genetic studies suggested that another class of GPCRs plays an important role in islet function. Pregnancy is characterized by an increase in insulin resistance; specifically, the effect of insulin at the level of the cell is decreased. Because of this, scientists reasoned that pregnancy would serve as a good model for understanding how beta cells adapt to insulin resistance. During pregnancy, islets respond by secreting more insulin to overcome this resistance. This is accomplished largely via an increase in the size and number of beta cells in the pancreas. As type 2 diabetes is a disorder in which islets do not fully adapt to insulin resistance, the implications of these studies may be applicable to understanding the pathophysiology of type 2 diabetes. To address this question, Kim et al. compared the level of expression of genes in islets from pregnant and nonpregnant female mice. These studies identified serotonin and its receptors as being important in regulating islet function during pregnancy. More specifically, these researchers observed that pancreatic beta cells express higher levels of serotonin receptors 2b and 1d during pregnancy, and signaling through these receptors mediates, in part, the pregnancy-induced increase in the number of pancreatic beta cells.

