F Integrated Physiology Of Insulin Resistance And Experimental Considerations
Interpretation of glucose tolerance tests requires measurement of plasma insulin concentrations. A: diet-induced obese and prediabetic nonobese diabetic mice both display glucose intolerance compared with lean chow-fed control mice, but the causes differ. The diet-induced obese mice mount a normal or even heightened insulin secretory response but are hyperglycemic owing to insulin resistance. The prediabetic NOD mice are glucose intolerant owing to defective insulin secretion. B: improved glucose tolerance can similarly result from increased insulin sensitivity or from increased insulin secretion .
Effective methods for in vivo assessment of insulin control of lipid metabolism are also available. Insulin suppression of lipolysis can be traced during hyperinsulinemic-euglycemic clamp studies using labeled palmitate and glycerol . Insulin control of hepatic de novo lipogenesis is a transcriptionally mediated effect and therefore cannot be assessed in acute infusion studies, but effective tracer methods, such as deuterated water supplementation, enable measurement of DNL over a period of several days and reveal decreased DNL in insulin-resistant models . A commonly employed protocol to measure insulin upregulation of the lipogenic transcriptional program is fasting refeeding . It is important to note, however, that insulin is not the only relevant variable in such studies nutrient activation of mTOR is a major consideration as are changes in other hormones .
A What Is Insulin Resistance
Insulin resistance in dose-response curves. A: in a hypothetical cell with decreased surface insulin receptor content, the dose-response curve is right-shifted but the maximal biological response is not decreased unless > 90% of surface receptors are lost. B: in a cell with an insulin signal transduction defect, or a combined receptor/post-receptor defect, both a right shift and decreased maximal response are observed. The right graph typifies human obesity-associated insulin resistance in muscle, liver, and adipose tissues.
What Is The Difference Between Glucagon And Insulin
Glucagon and insulin are both important hormones that play essential roles in regulating your blood glucose . Both hormones come from your pancreas alpha cells in your pancreas make and release glucagon, and beta cells in your pancreas make and release insulin.
The difference is in how these hormones contribute to blood sugar regulation. Glucagon increases blood sugar levels, whereas insulin decreases blood sugar levels. If your pancreas doesnt make enough insulin or your body doesnt use it properly, you can have high blood sugar , which leads to diabetes.
Read Also: Glucagon Deficiency Symptoms
Regulation Of Blood Glucose
Regulation of glucose in the body is done autonomically and constantly throughout each minute of the day. Normal BG levels should be between 60 and 140 mg/dL in order to supply cells of the body with its required energy. Brain cells dont require insulin to drive glucose into neurons however, there must still be normal amounts available. Too little glucose, called hypoglycemia, starves cells, and too much glucose creates a sticky, paralyzing effect on cells. Euglycemia, or blood sugar within the normal range, is naturally ideal for the bodys functions. A delicate balance between hormones of the pancreas, intestines, brain, and even adrenals is required to maintain normal BG levels.
D Selective Hepatic Insulin Resistance
Mechanisms for the development of nonalcoholic fatty liver disease despite hepatic insulin resistance. A: insulin normally activates de novo lipogenesis through sterol regulatory element binding protein 1c . B: the seemingly paradoxical coexistence of NAFLD and hepatic insulin resistance has spawned the hypothesis of selective hepatic insulin resistance, wherein insulin activation of lipogenesis is preserved despite impaired insulin regulation of glucose metabolism. However, hepatic de novo lipogenesis has multiple inputs, including ChREBP and mTORC1/SREBP-1c, both of which are activated in states of chronic overnutrition. Additionally, the primary pathway for hepatic triglyceride synthesis is re-esterification of preformed fatty acids, which are readily available in states of chronic overnutrition owing both to dietary supply and to adipose insulin resistance. Even if insulin receptor activation of SREBP-1c is impaired by hepatic insulin resistance, these other inputs are likely capable of supporting the lipogenic fluxes that lead to NAFLD. NEFA, nonesterified fatty acid WAT, white adipose tissue.
You May Like: Is Overnight Oats Good For Diabetics
What Severe Complications Can Occur Because Of Rationing Or Running Out Of Insulin
Diabetic ketoacidosis is an emergency condition that results if you dont have enough insulin to regulate your blood sugar. DKA causes your body to break down fat for energy in the absence of insulin. This leads to a dangerous accumulation of acids known as ketones in your blood that can cause your brain to swell and your body to go into shock.
Signs of diabetic ketoacidosis include:
- Thirst or a very dry mouth
- Frequent urination
- High levels of ketones in your urine
- Fatigue
- Nausea, vomiting, or stomach pain
- Difficulty breathing
- A fruity or acetone odor on your breath
- Confusion or acting drunk while sober
DKA is so common and can come on so quickly that it is the first sign of Type 1 diabetes in 20% of cases, and the way many type 1 diabetics are first diagnosed with the condition. If you go into diabetic ketoacidosis, dont try to hide it or make light of it. Treat it as the emergency it is and get to a hospital as soon as possible to recover. Ive had people tell me theyre tired of taking insulin, or that theyre rationing it due to cost. In type 1 diabetes, thats all it takes to end up in a life-threatening situation, says Dr. Zilbermint.
D Acylcarnitines Metabolic Inflexibility And Insulin Resistance
Acylcarnitines are measured in plasma to noninvasively probe inborn errors of FAO . The mechanisms of acylcarnitine appearance in plasma are incompletely understood, but plasma acylcarnitine concentrations are thought to reflect intracellular levels . Could plasma acylcarnitine levels thus be used as a biomarker for incomplete FAO in muscle? The major problem with this proposition is that plasma acylcarnitine levels are a function of FAO rates in not just skeletal muscle, but in all tissues. The liver in particular preferentially oxidizes lipids and is likely a major contributor to plasma acylcarnitine levels . Interestingly, in Pdk2/4/ mice with constitutive glucose oxidation in skeletal muscle, myocellular medium- and long-chain acylcarnitines were markedly decreased but plasma levels were unchanged compared with wild-type controls . This dissociation of muscle and plasma acylcarnitine profiles, also reported in humans , indicates that plasma acylcarnitines probably cannot be used to interrogate incomplete FAO in skeletal muscle, although several groups have attempted to do so .
Read Also: How Much Blood Sugar Is Too High
What Are The Symptoms Of Glucagon
Depending on the situation and condition, you can experience low and/or high blood sugar from abnormal glucagon levels.
Symptoms of low blood sugar
The signs and symptoms of low blood sugar include:
- Shaking or trembling.
- Weakness.
- Tingling or numbness in your face or mouth.
If youre experiencing these symptoms, its important to eat food with carbohydrates/sugar to treat it and bring your blood sugar levels up. If you experience these symptoms often, contact your healthcare provider.
Symptoms of high blood sugar
While high blood sugar levels are most commonly caused by an issue with not having enough insulin and not an isolated glucagon issue, its possible to have elevated blood sugar levels from rare glucagon issues. Early signs and symptoms of high blood sugar include:
- Increased thirst and/or hunger.
How Do You Take Insulin Without A Syringe
- Insulin pens look like large writing pens and can help prevent under- and overdosing. They also dont require refrigeration, are conveniently prefilled, and are more durable than syringes.
- Insulin pumps are attached to a thin tube thats implanted under your skin. Pumps are computerized or motorized, and some models also act as glucose monitors. They deliver insulin before each meal along with small amounts through the course of the day. In the US, about 60% of people with diabetes use some form of insulin pump.
- Jet injection devices are a good option if you hate needles. A jet injector holds several doses of insulin. After placing it against your skin, you press a button, and the insulin is pushed through.
- Inhalable insulin comes in a premeasured inhaler and was first approved in 2014. Its short-acting and usually not covered by insurance, which makes it more cost prohibitive than other types of insulin for most people with diabetes.
Unless you have an insulin pump that also works as a glucose monitor, insulin dosing is based on self-monitoring your blood glucose levels. You can check them by doing finger pricks or wearing a device that continuously monitors them for you.
Recommended Reading: Can Metformin Cause Joint Pain
What Conditions Are Related To Issues With Glucagon Function
People with diabetes can develop an inability to release enough glucagon in response to decreasing blood glucose levels. Because of this, theyre more likely to develop frequent low or severely low blood sugars if they take medication that could cause low blood sugars especially synthetic insulin and medications in the class of sulfonylurea.
People with Type 2 diabetes may have glucagon levels that are relatively higher than what would be considered normal based on blood glucose levels. This can contribute to higher blood sugars.
Glucagon production issues outside diabetes are uncommon, and some are rare. The following conditions can affect or be affected by your glucagon function:
Structural Analysis And Synthesis
Purified animal-sourced insulin was initially the only type of insulin available for experiments and diabetics. John Jacob Abel was the first to produce the crystallised form in 1926. Evidence of the protein nature was first given by Michael Somogyi, Edward A. Doisy, and Philip A. Shaffer in 1924. It was fully proven when Hans Jensen and Earl A. Evans Jr. isolated the amino acids phenylalanine and proline in 1935.
The amino acid structure of insulin was first characterized in 1951 by Frederick Sanger, and the first synthetic insulin was produced simultaneously in the labs of Panayotis Katsoyannis at the University of Pittsburgh and Helmut Zahn at RWTH Aachen University in the mid-1960s.Synthetic crystalline bovine insulin was achieved by Chinese researchers in 1965. The complete 3-dimensional structure of insulin was determined by X-ray crystallography in Dorothy Hodgkin‘s laboratory in 1969.
Two other Nobel Prizes have been awarded for work on insulin. British molecular biologist Frederick Sanger, who determined the primary structure of insulin in 1955, was awarded the 1958 Nobel Prize in Chemistry.Rosalyn Sussman Yalow received the 1977 Nobel Prize in Medicine for the development of the radioimmunoassay for insulin.
Recommended Reading: Can You Take Too Much Metformin
C Hepatic Insulin Signaling: Effectors And Effects
Insulin from the endocrine pancreas is secreted into the portal vein, so the liver is exposed to insulin concentrations two- to threefold higher than those in the general circulation . Portal venous insulin measurements, especially in rodents, are difficult and infrequently performed, but investigators studying hepatic insulin action by infusing insulin peripherally must keep in mind that the increment in plasma insulin concentration measured from a peripheral site is not equal to the increment in portal vein insulin concentration seen by the liver.
The pathway diversification of hepatic insulin signaling appears to occur largely distal to AKT activation. AKT substrates include GSK3 , the transcription factor forkhead box O1 , and multiple regulators of mTORC1 activity, which in turn control a large anabolic program upregulating lipogenic gene expression and protein synthesis . Although direct hepatocellular insulin signaling for metabolic control may not be entirely AKT-dependent, alternative pathways are yet to be described . The considerable functional redundancy between insulin signaling and nutrient sensing pathways, especially mTOR signaling, has challenged attempts to prove the existence of alternative insulin signaling pathways in hepatocytes . With this in mind, we now consider the aforementioned physiological branches of hepatocellular insulin signaling in turn.
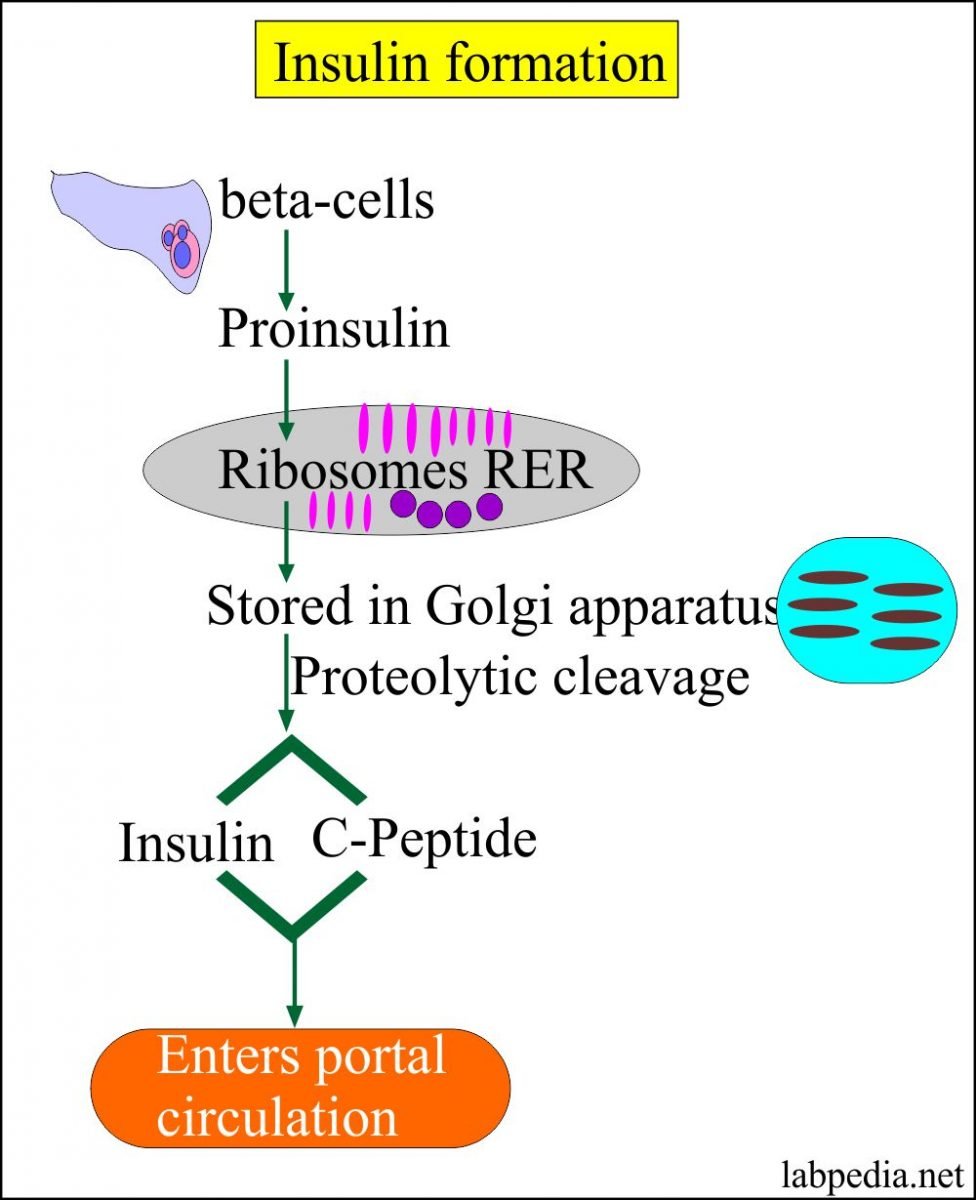
Iii Insulin Gene Transcription
The insulin gene on chromosome 11 is primarily expressed in pancreatic b cells, but is expressed in low levels in the brain, thymus, and in the yolk sak during fetal development . It has three exons and two introns, and its transcription results in the 446 base pair preproinsulin mRNA .
Figure 1. Various levels of glucose regulation of insulin gene expression. Glucose stimulates nuclear translocation of Pdx-1 promotes Pdx-1 and MafA phosphorylation and binding to the insulin promoter and stimulates transcription of the insulin gene, pre-mRNA splicing, translation, and mRNA stability. ).
Transcription of the insulin gene to preproinsulin mRNA is sophisticated and reflects the tight regulation by transcription factors and recruited coactivators. Pdx-1, NeuroD1 and MafA are important transcription factors in b cell function, respond to elevated glucose levels. Individual b cells respond to ambient glucose with differential insulin secretion, and these changes are apparent at the level of gene transcription . At the level of the islet, rapid increase in blood glucose results in rapid elevation in preproinsulin mRNA in the endocrine pancreas. A rapid decrease in blood glucose results in a slow decline in preproinsulin mRNA.
This is due to the unusual stability of preproinsulin mRNA, further stabilized by increased glucose concentrations . The specific regulation of this molecules translation is the primary mechanism of insulin production control .
Don’t Miss: Journal Articles On Type 2 Diabetes
Evolution And Species Distribution
Insulin may have originated more than a billion years ago. The molecular origins of insulin go at least as far back as the simplest unicellular eukaryotes. Apart from animals, insulin-like proteins are also known to exist in the Fungi and Protista kingdoms.
Insulin is produced by beta cells of the pancreatic islets in most vertebrates and by the Brockmann body in some teleost fish.Cone snailsConus geographus and Conus tulipa, venomous sea snails that hunt small fish, use modified forms of insulin in their venom cocktails. The insulin toxin, closer in structure to fishes’ than to snails’ native insulin, slows down the prey fishes by lowering their blood glucose levels.
C Adipokines And Hepatokines In Insulin Resistance
The explosion of newly identified secreted peptide hormones in the past 20 yr calls to mind the early 20th century, when investigators in the nascent discipline of endocrinology were rewarded with the discoveries of insulin, sex steroids, thyroxine, and other fundamental hormones . The identification of new hormones, now as then, is often received with great excitement and therapeutic hope. For many new hormones, however, pathophysiological significance and therapeutic potential are still uncertain. Here, we briefly highlight several of the best-studied new hormones, focusing on the evidence for their role in human insulin resistance.
In this review, we have highlighted the role of WAT and liver as master controllers of substrate storage and delivery. However, they are also prolific endocrine organs, and several adipokines and hepatokines have been implicated in human insulin resistance. Here we limit our discussion to retinol binding protein 4 , adiponectin, fetuin-A , and FGF21, four circulating mediators with particularly strong evidence of relevance to human insulin resistance.
You May Like: Insulin Is Secreted In Response To
What Causes Someone To Be Prescribed Insulin
If your body doesnt make insulin or doesnt make enough, you are eventually diagnosed with type 1 diabetes. It used to be called juvenile diabetes, but new estimates show that as many as half of people with type 1 diabetes are not diagnosed until adulthood. On the other hand, if your body doesnt use insulin properly, you have type 2 diabetes.
While people with type 1 diabetes need to take insulin to survive, many people with type 2 are able to stave off insulin use or even avoid it altogether by exercising, losing weight, adapting healthier eating habits, or using other prescription medications.
Can I Have A Negative Reaction To Insulin
One complication facing people with diabetes who use insulin is the potential for severe hypoglycemia, also known as insulin shock, which involves using too much insulin and causing your blood sugar to drop extremely low. This can cause coma, seizures, and heart attacks, says Dr. Powers. It requires treatment in a hospital but thankfully is highly treatable once you are there.
Also Check: What Color Is The Diabetes Awareness Ribbon
How Should I Store My Insulin
- Keep current insulin at room temperature to help alleviate injection discomfort.
- Insulin can usually be stored at room temperature for about a month. Once in use, insulin pens should be stored at room temperature. Expiration dates of insulin pens can vary depending upon the type of insulin. For disposable pens, you should discard the entire device when empty or when you reach the expiration date.
- Store extra insulin in the refrigerator.
- Dont expose insulin to excessive cold or heat.
Vi Regulation Of Insulin Release
Insulin release from pancreatic b cells is tightly regulated, and allows the sensitive response of insulin levels to calorigenic nutrients in the body. Glucose, free fatty acids, and amino acids serve as fuel stimuli for insulin release, promoting insulin granule exocytosis. Additional hormonal factors influence the regulation pathway. Pharmacological agents can also be used to augment insulin release.
A. Glucose-stimulated insulin secretion
Figure 5. Hyperglycemic clamp illustration. Example of hyperglycemic clamp testing in obese adolescents with normal glucose tolerance , impaired glucose tolerance , and type 2 diabetes . In the hyperglycemic clamp in healthy, non-diabetic individuals, glucose concentration is briskly elevated by administering a suitable intravenous glucose infusion at time 0. This elicits a rapid and short-lived insulin secretion peak due to release of preformed insulin vesicles, followed by a drop towards basal levels and then by a relatively rapid return to a sustained increase in insulin in the second half of the clamp as dextrose infusion is continued. This example illustrates the loss, in first and second phase insulin secretion, as individual progress from normal to impaired glucose tolerance, to type 2 diabetes. In the latter, the first phase insulin response is essentially lost and the second phase insulin response is reduced. ).
B. Proteins and Amino Acids
C. Lipids and Free Fatty Acids
D. Incretin Hormones
Read Also: Glucose Definition Medical

