B Are There Local Factors Making
Many T1D target Ag are expressed by all islet cells and by other neuroendocrine lineages. This is the case for GAD , IA-2 , and chromogranin A , whereas expression of PI, IGRP, and ZnT8 is largely, although not exclusively, restricted to -cells. Despite this broad Ag distribution, the autoimmune destruction of -cells is quite selective. -Cells constitute 6070% of total islet cells. They are surrounded by or interspersed with , , and PP cells secreting glucagon, somatostatin, and pancreatic polypeptide, respectively. Although several autoimmune diseases can be elicited by immunization against self-tissues in adjuvant , it has not been possible to induce diabetes by immunizing animals with islets. Similarly, insulin B9-23 peptide immunization induces experimental autoimmune diabetes only in transgenic Balb/c mice expressing the costimulatory molecule B7.1 in their islets . These observations suggest that local signals within the islets may be required for full-blown diabetes to develop and that this requirement can be bypassed by transgenic B7.1 expression . The role of local factors in the disease process is further underscored by the striking heterogeneity of islet infiltrates among and within individual pancreata .
Production Of Digestive Enzymes
The pancreas produces secretions necessary for you to digest food. The enzymes in these secretions allow your body to digest protein, fat and starch from your food. The enzymes are produced in the acinar cells which make up most of the pancreas. From the acinar cells the enzymes flow down various channels into the pancreatic duct and then out into the duodenum. The secretions are alkaline to balance the acidic juices and partially digested food coming into the duodenum from the stomach.
C The Genetic Background
Gene polymorphisms set the autoimmune potential of a given individual by regulating immune tolerance mechanisms at the central level and in the periphery , which subsequently affect generation and survival of autoreactive T lymphocytes . Indeed, most T1D susceptibility loci unraveled by genome-wide association studies map to genes influencing immune function . Although multiple genes contribute to T1D susceptibility, each one taken individually carries a weak effect. The only exception is the HLA locus, which provides the largest contribution to disease risk .
1 From genes to immune mechanisms: central tolerance
Among the numerous gene variants that have been associated to T1D, the susceptibility alleles that confer the strongest predisposition are MHC class II variants, which influence the way in which epitopes are presented to CD4+ T cells. The MHC class II locus contributes approximately 40% of genetic susceptibility to T1D. T1D-susceptible MHC class II alleles carry a serine, an alanine, or a valine residue at position 57 of the DQ chain . Class II alleles carrying an aspartic acid at position 57 are neutral or confer dominant protection . Position 57 is critical for epitope binding and formation of peptide-HLA complexes. In the NOD mouse, the class II allele I-Ag7 associated with diabetes shows structural homology with human DQ susceptibility alleles .
2 From genes to immune mechanisms: peripheral tolerance
You May Like: What Inhibits Insulin Secretion
What Else Should I Know About Insulin
Our insulin requirements vary depending on the food we eat, how much activity we do, our general and mental health, how much we currently weigh, and our age.
Talk to your doctor about how you should adjust your insulin dose when you:
- Are prescribed other medications that may affect your body’s sensitivity to insulin
- Are unwell or have an infection
- Feel stressed or exceptionally tired
- Gain or lose weight
- Reduce or increase your activity levels
- Are trying for a baby or find out you are pregnant.
You should also regularly monitor your blood glucose levels and be able to count your calories and know how to calculate how much insulin to take and when to adjust it. If you don’t know how to do this, ask your doctor or talk to a diabetes nurse.
You should know what to do if your blood sugar level goes too high or too low and have an action plan in place so that your family and friends also know what to do.
Most insulin in the U.S. is sold as a concentration of 100 units per ml . However, there are currently four concentrated products on the market:
- Humulin R which has a 500 units per ml strength available
- Humalog which has a 200 units per ml strength available
- Toujeo Solostar which contains 300 units per ml of insulin glargine
- Tresiba which has a 200 units per ml strength available.
A Pi Is The Initiating
Besides PI, glutamic acid decarboxylase , tyrosine phosphatase-like islet-cell Ag 2 , zinc transporter 8 , islet glucose-6-phosphatase catalytic subunit-related protein , and chromogranin A have been identified as key Ag of -cell autoimmunity because they are the molecular targets of autoreactive T cells and/or aAb. A popular tenet is that PI is the only T1D Ag exclusively expressed by -cells. However, low level expression is also found in mTEC involved in thymic education, in circulating myeloid cells , in DC subsets , and in the rat embryonic central and peripheral nervous system .
A favored adagio in autoimmunity is that there could be one primary self-Ag that initiates pathogenesis. Tissue destruction through targeting of this Ag could further release other ones, thus amplifying the autoimmune cascade through a phenomenon known as epitope spreading. In the NOD mouse, PI has been identified as the initiating -cell Ag. The importance of insulin is supported by data on insulin knockout NOD mice. Different from humans, rodents express two insulin isoforms, referred to as insulin 1 and 2. We generated NOD mice defective for the Ins2 gene, which is the prevalent isoform in the thymus. These mice display accelerated T1D , likely related to defective deletion of PI-reactive T cells . Conversely, NOD mice defective for the Ins1 gene, which is expressed in the islets along with Ins2 and is thus targeted by T cells, are less susceptible to T1D .
Don’t Miss: Management Of High Blood Sugar
Evolution And Species Distribution
Insulin may have originated more than a billion years ago. The molecular origins of insulin go at least as far back as the simplest unicellular eukaryotes. Apart from animals, insulin-like proteins are also known to exist in the Fungi and Protista kingdoms.
Insulin is produced by beta cells of the pancreatic islets in most vertebrates and by the Brockmann body in some teleost fish.Cone snailsConus geographus and Conus tulipa, venomous sea snails that hunt small fish, use modified forms of insulin in their venom cocktails. The insulin toxin, closer in structure to fishes’ than to snails’ native insulin, slows down the prey fishes by lowering their blood glucose levels.
Mechanisms Of Insulin Resistance
Physiologically, at the whole body level, the actions of insulin are influenced by the interplay of other hormones. Insulin, though the dominant hormone driving metabolic processes in the fed state, acts in concert with growth hormone and IGF-1 growth hormone is secreted in response to insulin, among other stimuli, preventing insulin-induced hypoglycaemia. Other counter-regulatory hormones include glucagon, glucocorticoids and catecholamines. These hormones drive metabolic processes in the fasting state. Glucagon promotes glycogenolysis, gluconeogenesis and ketogenesis. The ratio of insulin to glucagons determines the degree of phosphorylation or dephosphorylation of the relevant enzymes. Catecholamines promote lipolysis and glycogenolysis glucocorticoids promote muscle catabolism, gluconeogenesis and lipolysis. Excess secretion of these hormones may contribute to insulin resistance in particular settings, but does not account for the vast majority of insulin resistant states.
Read Also: Does Metformin Increase Insulin Secretion
Insulin Secretion Diabetes And Ketone Body Metabolism
Insulin secretion occurs through two mechanisms. The first is a process that involves closing of the cell-surface ATP-sensitive potassium channels in response to increases in the circulating glucose concentrations, which stimulates exocytosis of insulin-containing vesicles from the -islet cells through an increase in the cytosolic calcium concentration. The second mechanism is dependent on pyruvate carboxylase, which is highly expressed in -islet cells it has been estimated that 3545% of pyruvate enters the citric acid cycle through this anaplerotic pathway in -islet cells. Inhibition of pyruvate carboxylase with phenylacetic acid decreases glucose-stimulated insulin release from -islet cells. Furthermore, there is evidence that pyruvate carboxylase plays an important role in the early stages of type 2 diabetes. Specifically, in Zucker fatty rats with insulin resistance, the hyperfunctioning -islet cells increase insulin production in part through increases in pyruvate carboxylase activity.
Sleep And Sleep Deprivation
Acute sleep deprivation in healthy young adults has been reported to raise fasting blood glucose concentrations in association with altered diurnal cortisol secretion and reduced heart rate variability. These effects suggest increased counter-regulatory hormone secretion via hyper-arousal with activation of the hypothalamo-pituitary adrenal axis. There is also accumulating evidence that chronic sleep deprivation may impact on insulin and insulin resistance. Recent epidemiological studies report that reduced sleep duration is associated with increased BMI. Sleep deprivation is associated with decreased plasma concentrations of leptin, the adipocyte peptide hormone regulating fat mass and appetite, and increased concentrations of ghrelin, which increases appetite. Growth hormone is secreted during slow wave sleep, sleep declines with age and growth hormone deficiency in adults has been associated with central adiposity and insulin resistance, but whether sleep deprivation acts through these mechanisms is not clearly established. Obstructive sleep apnoea , where sleep disturbance results from obstruction to breathing during sleep, is associated with impaired glucose tolerance independent of adiposity, and improves with continuous positive airway pressure treatment but whether this is due to resolution of hypoxia and hypercapnia, or to effects on sleep quality, is unclear.
Read Also: Metformin And Water Retention
Viii Pharmacologic Modulators Of Insulin Response
There is a plethora of pharmcologic agents designed to target various aspects of glucose metabolism. In this chapter, we provide examples of pharmacologic agents that directly or indirectly modulate insulin response.
A. Incretin mimetics
Diabetes therapeutics have recently utilized the role of incretin hormones for pharmacologic benefit. Due to the desirable effect of GLP-1 on hemoglobin A1c reduction and weight loss , GLP-1 receptor agonists and inhibitors of its degradation via dipeptidyl peptidase-4 inhibitors, have been used to treat type 2 diabetes since 2005.
Short-acting GLP-1 receptor agonists , and long-acting GLP-1 receptor agonists potentiate insulin secretion and reduce gastric motility . Given that GLP-1 receptor agonists potentiate glucose-induced insulin gene transcription, they, alone, do not induce hypoglycemia when used as monotherapy .
DPP-4 inhibitors can significantly increase the peak post-prandial concentration of GLP-1 . Additionally, sitagliptin has been found to potentiate GSIS independently of GLP-1 via islet peptide tyrosine tyrosine .
B. Sulfonylureas
C. Insulin Sensitizers
D. Diazoxide
Diazoxide is a sulfonamide pharmacological agent used in treatment of hyperinsulinism, insulinoma, and hypoglycemia due to overtreatment with sulfonylureas. It works by opening b cell membrane potassium ATP channels, hyperpolarizing the b cells, thus decreasing intracellular calcium concentration and inhibiting insulin secretion .
Types Of Insulin Treatments
All types of insulin produce the same effect. They are used to mimic the natural increases and decreases of insulin levels in the body during the day. The makeup of different types of insulin affects how fast and how long they work.
The type of insulin youll be prescribed will vary depending on things like:
- your age
- how long it takes your body to absorb insulin
- how long insulin stays active in your system
| Insulin type | ||
|---|---|---|
| varied peaks | 10 to 16 hours | Taken twice a day, commonly 10 to 30 minutes before breakfast and dinner. This type is a combination of intermediate- and short-acting insulin. |
Talk with a doctor about the right insulin for you and your lifestyle.
Also Check: How Many Carbs Per Day For Diabetics
Insulin Receptors And Insulin Binding
Insulin mediates its actions through binding to insulin receptors. The insulin receptor was first characterised in 1971. It consists of a heterotetramer consisting of 2 and 2 glycoprotein subunits linked by disulphide bonds and is located on the cell membrane. The gene coding for the insulin receptor is located on the short arm of chromosome 19. Insulin binds to the extracellular subunit, resulting in conformational change enabling ATP to bind to the intracellular component of the subunit. ATP binding in turn triggers phosphorylation of the subunit conferring tyrosine kinase activity. This enables tyrosine phosphorylation of intracellular substrate proteins known as insulin responsive substrates . The IRS can then bind other signalling molecules which mediate further cellular actions of insulin.
PI 3-kinase promotes the translocation of glucose transporter proteins, glycogen, lipid and protein synthesis, anti-lipolysis and the control of hepatic gluconeogenesis. PI 3-kinase acts via serine and threonine kinases such as Akt/protein kinase B , protein kinase C and PI dependent protein kinases1& 2 . The RAS pathway activates transcription factors and stimulates the growth promoting actions of insulin. Thus broadly, PI 3-kinase mediates insulins metabolic effects, e.g. cellular glucose uptake, while RAS significantly mediates insulins mitogenic effects, together with other less well described actions. These pathways are presented schematically in .
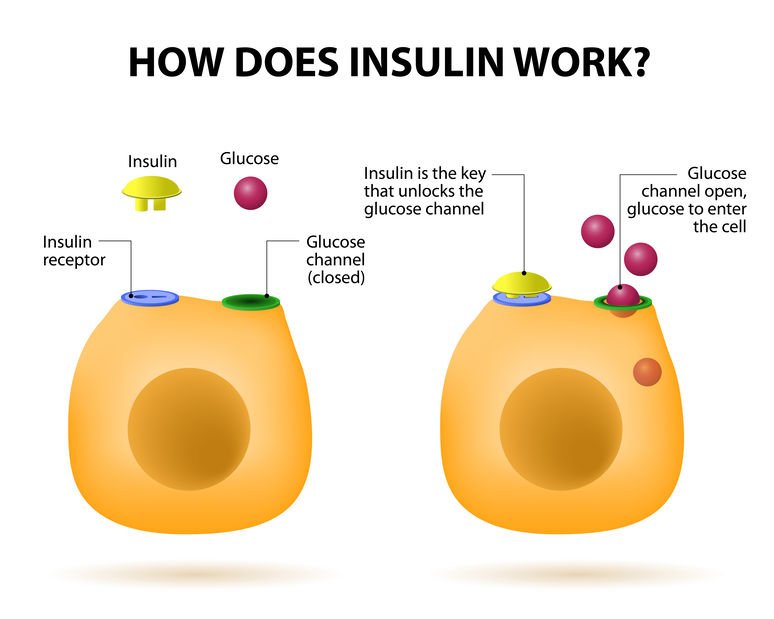
Insulin Blood Sugar And Type 2 Diabetes
Insulin is a key player in developing type 2 diabetes. This vital hormoneyou cant survive without itregulates blood sugar in the body, a very complicated process. Here are the high points:
- The food you eat is broken down into blood sugar.
- Blood sugar enters your bloodstream, which signals the pancreas to release insulin.
- Insulin helps blood sugar enter the bodys cells so it can be used for energy.
- Insulin also signals the liver to store blood sugar for later use.
- Blood sugar enters cells, and levels in the bloodstream decrease, signaling insulin to decrease too.
- Lower insulin levels alert the liver to release stored blood sugar so energy is always available, even if you havent eaten for a while.
Thats when everything works smoothly. But this finely tuned system can quickly get out of whack, as follows:
- A lot of blood sugar enters the bloodstream.
- The pancreas pumps out more insulin to get blood sugar into cells.
- Over time, cells stop responding to all that insulintheyve become insulin resistant.
- The pancreas keeps making more insulin to try to make cells respond.
- Eventually, the pancreas cant keep up, and blood sugar keeps rising.
Don’t Miss: How Much Metformin Is Too Much
Iii Insulin Gene Transcription
The insulin gene on chromosome 11 is primarily expressed in pancreatic b cells, but is expressed in low levels in the brain, thymus, and in the yolk sak during fetal development . It has three exons and two introns, and its transcription results in the 446 base pair preproinsulin mRNA .
Figure 1. Various levels of glucose regulation of insulin gene expression. Glucose stimulates nuclear translocation of Pdx-1 promotes Pdx-1 and MafA phosphorylation and binding to the insulin promoter and stimulates transcription of the insulin gene, pre-mRNA splicing, translation, and mRNA stability. ).
Transcription of the insulin gene to preproinsulin mRNA is sophisticated and reflects the tight regulation by transcription factors and recruited coactivators. Pdx-1, NeuroD1 and MafA are important transcription factors in b cell function, respond to elevated glucose levels. Individual b cells respond to ambient glucose with differential insulin secretion, and these changes are apparent at the level of gene transcription . At the level of the islet, rapid increase in blood glucose results in rapid elevation in preproinsulin mRNA in the endocrine pancreas. A rapid decrease in blood glucose results in a slow decline in preproinsulin mRNA.
This is due to the unusual stability of preproinsulin mRNA, further stabilized by increased glucose concentrations . The specific regulation of this molecules translation is the primary mechanism of insulin production control .
Talk With Your Doctor
Knowing how your body works can help you stay healthy. Insulin and glucagon are two critical hormones your body makes to keep your blood sugar levels balanced. Its helpful to understand how these hormones function so you can work to avoid diabetes.
If you have more questions about insulin, glucagon, and blood glucose, talk to your doctor. Questions you have might include:
- Is my blood glucose at a safe level?
- Do I have prediabetes?
Also Check: Hypertriglyceridemia Low Carbohydrate Diet
Vphysiology Of Insulin Secretion In Vivo
Insulin secretionin vivo has also been extensively studied. As predictable from the studies of single beta cells described above, the most important regulators of insulin secretion are circulating nutrients, in particular, glucose. In the fasting state, insulin secretion is maintained at levels that provide sufficient insulin to constrain hepatic glucose release at rates that match glucose utilization and so the plasma glucose concentration is maintained at normal levels of 90 mg/dl . After meal ingestion, glucose concentrations in the circulation rise and stimulate insulin secretion . Increased delivery of insulin into the circulation causes further suppression of hepatic glucose release and increased stimulation of glucose uptake by insulin-sensitive tissues such as muscle to restore normoglycemia. Therefore, the simplest model to describe insulin secretion in vivo would have two components: a constant basal rate of insulin secretion superimposed on which are meal-related increments. Although this model is commonly employed by physicians attempting to replace insulin in patients who secrete insufficient insulin, it is an oversimplification of a very complex dynamic neuroendocrine secretory system.
Juris J. Meier, in, 2016
Insulin And Blood Glucose Levels
Insulin helps control blood glucose levels by signaling the liver and muscle and fat cells to take in glucose from the blood. Insulin therefore helps cells to take in glucose to be used for energy.
If the body has sufficient energy, insulin signals the liver to take up glucose and store it as glycogen.
The liver can store up to around 5% of its mass as glycogen.
Some cells in the body can take glucose from the blood without insulin, but most cells do require insulin to be present.
Read Also: Signs That Your Blood Sugar Is High
Exercise & Physical Activity
Since Chauveu & Kaufmans remarkable observation in 1887 that When a horse chews on hay the concentration of glucose in the blood draining its masseter muscle substantially decreases a large body of evidence supports the role of exercise in improving insulin sensitivity and its beneficial outcomes in insulin resistant states. Epidemiological studies such as the US Physicians Health Study have reported substantial decreases in the relative risk of type 2 diabetes with lifelong regular physical activity. Large scale randomised controlled clinical trials such as the Diabetes Prevention Program and the Finnish Prevention Study demonstrate a 58% reduction in progression of impaired glucose tolerance to type 2 diabetes by intensive lifestyle modification which included a minimum of 2030 minutes of exercise per day. Acute exercise increases GLUT 4 translocation to sarcolemmal membrane, whereas chronic exercise training increases Glut 4 mRNA expression. In addition to this insulin-dependent mechanism, enhanced glucose uptake into exercising muscle occurs by multiple insulinin dependent mechanisms. Exercise training appears to enhance insulin sensitivity by increased post-receptor insulin signalling increased insulin-mediated glucose transport appears to be related to enhanced signal transduction at the level of IRS proteins and PI 3-kinase.

