Glucose Sensing As A Distributed System
The above sensing mechanisms are activated by rises in glucose concentrations to induce glucose utilization and storage to prevent excessive upwards excursions of glycemia in the absorptive phase. This is achieved by adapting the metabolic activity of cells, organs and whole body through transcriptional control of gene expression, regulation of enzyme activities, hormone secretion and regulation of autonomic nervous activity.
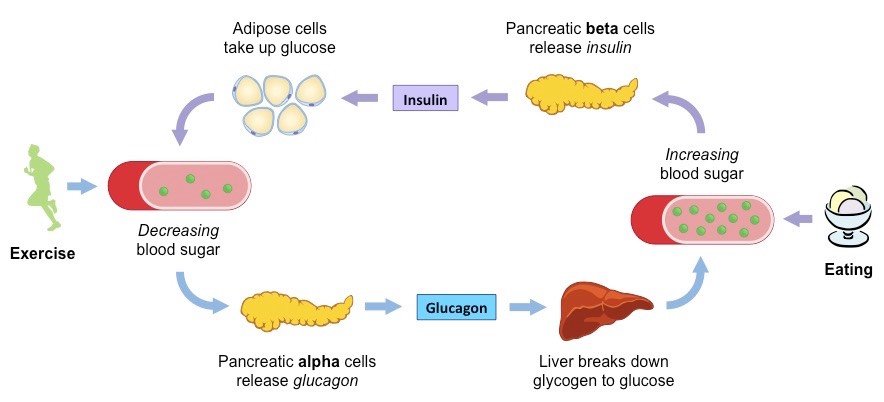
Equally important is the response to hypoglycemia, which is not only the suppression of the hyperglycemic responses but also a set of positive counterregulation mechanisms aimed at preventing a fall in blood glucose concentrations that could otherwise threaten brain function and survival of the individual. A critical response is the stimulated secretion of glucagon, epinephrine and norepinephrine that quickly mobilizes hepatic glycogen and, over longer fasting periods, also stimulates de novo glucose synthesis.
Additional Islet Hormones Are Observed In Some Cells Co
Given that early endocrine cells are capable of producing more than a single hormone, we investigated the possibility that cells co-producing insulin and glucagon also produce other islet hormones. Somatostatin and PP were detected in cells co-producing insulin and glucagon. At 9 weeks, the frequency of these triple-positive cells was < 1 in 1,000 . By 15 weeks, somatostatin and PP production was restricted to separate cell populations, thus resembling the pattern in adult islets. In contrast, ghrelin immunoreactivity did not co-localise with insulin and/or glucagon immunoreactivity at any of the examined developmental time points , suggesting a separate cell ancestry for this rare islet cell type.
Fig. 3
Other islet hormones in cells producing insulin and/or glucagon during human pancreas development. Triple immunofluorescence staining for insulin , glucagon , and somatostatin , PP or ghrelin in human fetal and adult pancreas sections. White asterisks denote regions enlarged in the upper-right corner of each image. Scale bars, 100 m
Consistent with observations made over 30 years ago , we observed two distinct islet architectures with respect to PP production in the developing human pancreas . Thus while PP production was limited to a few cells in the majority of islets, some fetal islets were comprised almost entirely of PP-producing cells that did not co-produce insulin or glucagon .
The Precious Pancreas: Insulin Glucagon And Digestive Juices
Posted on 10/10/19 by Adelaide Elkin
Located behind the stomach and at the back of the abdomen is the six-inch-long, tadpole-shaped pancreas. From head to tail, the pancreas extends across the abdomen. The head is based where the stomach meets the duodenum .
The pancreas in context. Image from Human Anatomy Atlas.
The pancreas is an accessory digestive organ made of small glandular clusters of epithelial cells. Its crucial for converting food to fuel for body cells and contains both endocrine and exocrine gland cells.
Read Also: Can Diabetics Eat Macaroni And Cheese
Lactate: Arterial Levels And Net Hepatic Balance
In the control group, arterial blood lactate levels rose modestly due to an increase in net hepatic lactate output during hyperglycemia . When glucagon was increased, the arterial blood lactate level rose as in the control group, also due to an increase in net hepatic lactate production. However, with glucagon, the rise in net hepatic lactate output and the lactate level occurred more quickly, presumably resulting from the hormone’s effect on glycogenolysis. When epinephrine was increased, arterial lactate levels rose to a markedly greater extent than in C or G despite the fact that net hepatic output essentially ceased within 30 min. This indicates that the catecholamine stimulated the net release of lactate from nonhepatic tissues . Finally, when both hormones were increased concurrently, there was a brief increase in net hepatic lactate output and a resulting rise in the blood lactate level, followed by a fall in net hepatic lactate output to zero and a continued rise in the lactate level to almost 2.5 mmol/l.
Quantification Of Cells Co
To characterise the production of insulin and glucagon during human pancreas development, we performed a detailed quantitative analysis of insulin and glucagon immunoreactivity in the fetal pancreas between 9 and 21 weeks, and in the adult pancreas . During this critical window of development, the pattern of insulin and glucagon staining in the pancreas evolves from a smattering of insulin-producing cells to cohesive islet structures similar to those found in the adult organ. Despite a gross morphological change, the proportion of cells producing insulin and/or glucagon remained relatively constant throughout this period of development. At 9 to 10 weeks, these endocrine cells accounted for 1.31±0.31% of the total pancreatic cell population at 21 weeks, this population was 2.69±0.88% . In the adult pancreas, 1.38±0.31% of cells within the pancreas were immunoreactive for insulin and/or glucagon .
Fig. 1
Insulin- and/or glucagon-producing cells during human pancreas development. Double immunofluorescence staining for insulin and glucagon in human fetal and adult pancreas sections. Representative merged images are shown for each age co-localisation of insulin and glucagon is in yellow. White asterisks denote regions enlarged in the upper-right corner. Scale bars, 100 m
Fig. 2
You May Like: Can Diabetics Eat Macaroni And Cheese
Molecular Identification Of The Specimen
The toothed whale specimen was measured 2.73 m and suggested to be a female pygmy sperm whale or a dwarf sperm whale , based upon the external physical and morphological examination . To further identify the species, we successfully amplified and sequenced for the three mitochondrial regions from genomic DNA, the 5 end of the cox1 gene , the partial cytb gene , and the complete D-loop sequence . The cox1 sequence submitted to the BOLD Systems matched to pygmy sperm whale reference sequences with 100% similarity . The DNA Surveillance analysis results showed the present specimen sequences clustered with pygmy sperm whale reference sequences for both D-loop and the cytb with high bootstrap support . According to literature and our anatomical observation, as well as the molecular information, we identified that the whale was an adult female pygmy sperm whale .
Cells And Secretions Of The Pancreatic Islets
The pancreatic islets each contain four varieties of cells:
- The alpha cell produces the hormone glucagon and makes up approximately 20 percent of each islet. Glucagon plays an important role in blood glucose regulation low blood glucose levels stimulate its release.
- The beta cell produces the hormone insulin and makes up approximately 75 percent of each islet. Elevated blood glucose levels stimulate the release of insulin.
- The delta cell accounts for four percent of the islet cells and secretes the peptide hormone somatostatin. Recall that somatostatin is also released by the hypothalamus , and the stomach and intestines also secrete it. An inhibiting hormone, pancreatic somatostatin inhibits the release of both glucagon and insulin.
- The PP cell accounts for about one percent of islet cells and secretes the pancreatic polypeptide hormone. It is thought to play a role in appetite, as well as in the regulation of pancreatic exocrine and endocrine secretions. Pancreatic polypeptide released following a meal may reduce further food consumption however, it is also released in response to fasting.
Read Also: What Drugs Should Not Be Taken With Metformin
Identification And Characterization Of Pygmy Sperm Whale Insulin
Two exons and one intron in the pygmy sperm whale insulin gene locus were obtained from genomic DNA, which contained the CDS of 333 bp encoding a 110 amino acid preproinsulin protein . The nucleotide and deduced amino acid sequence has been deposited in GenBank . The preproinsulin contains signal peptide of , B-chain , C-peptide , and A-chain . Overall, preproinsulin was highly conserved compared with other mammalian orthologs . The identity of pygmy sperm whale preproinsulin with other vertebrates preproinsulin orthologs was 93.6% , 93.6% , 88.2% , 87.3% , 86.4% , 79.1% , 62.8% , 59.3% , 53.9% , and 43.1% . The mature pygmy sperm whale insulin peptide, A-chain and B-chain, was highly conserved. The greatest sequence identity was observed in the B-chain, being 84.0~100% identical among different species, with the identity in the A-chain at 66.7~100%. However, the C-peptide was more variable among different vertebrate preproinsulin orthologs.
We next performed phylogenetic analysis using the sequences of 21 vertebrates preproinsulin obtained from GenBank. A phylogenetic tree generated by the neighbor-joining method revealed that the pygmy sperm whale preproinsulin forms a cluster with the cetacean predicted preproinsulin with high bootstrap support value, suggesting that the protein was indeed the ortholog of the cetacean preproinsulin .
Talk With Your Doctor
Knowing how your body works can help you stay healthy. Insulin and glucagon are two critical hormones your body makes to keep your blood sugar levels balanced. Its helpful to understand how these hormones function so you can work to avoid diabetes.
If you have more questions about insulin, glucagon, and blood glucose, talk to your doctor. Questions you have might include:
- Is my blood glucose at a safe level?
- Do I have prediabetes?
Recommended Reading: How Do You Feel When Blood Sugar Is High
Insulin And Glucagon Influences On Glucose Homeostasis
Ktorza and associates12 evaluated insulin and glucagon secretion during the perinatal period, and noted that insulin and glucagon are detected in most species early in gestation. The insulin-glucagon molar ratio is high in the fetus at term, but then decreases after birth and remains low during the first hours after birth. This change favors glycogenolysis and gluconeogenesis after birth. King and colleagues13 studied postnatal development of insulin secretion in the premature neonate for 110 days after birth and in the term neonate for up to 47 days after birth. Insulin levels were measured before and 30 minutes after administration of glucose parenterally or enterally. Premature neonates exhibited a small increase in insulin secretion in response to glucose administration on postnatal day 1, which gradually increased over the course of the study. The term neonate secreted more insulin than preterm infants. The investigators concluded that the premature neonate may take up to 18 weeks to develop a fully mature response to an increase in plasma glucose concentration.
Epinephrine Cortisol And Growth Hormone:
Epinephrine, cortisol, and growth hormone are other hormones that help maintain blood sugar levels. They, along with glucagon are called stress or gluco-counter-regulatory hormones which means they make the blood sugar rise.
Epinephrine is released from nerve endings and the adrenals, and acts directly on the liver to promote sugar production . Epinephrine also promotes the breakdown and release of fat nutrients that travel to the liver where they are converted into sugar and ketones.
Cortisol is a steroid hormone also secreted from the adrenal gland. It makes fat and muscle cells resistant to the action of insulin, and enhances the production of glucose by the liver. Under normal circumstances, cortisol counterbalances the action of insulin. Under stress or if a synthetic cortisol is given as a medication , cortisol levels become elevated and you become insulin resistant. When you have Type 1 diabetes, this means your may need to take more insulin to keep your blood sugar under control.
Growth Hormone is released from the pituitary, which is a part of the brain. Like cortisol, growth hormone counterbalances the effect of insulin on muscle and fat cells. High levels of growth hormone cause resistance to the action of insulin.
Don’t Miss: Which Pancreatic Cells Release Insulin And Glucagon
What Happens If I Have Too Little Glucagon
Unusual cases of deficiency of glucagon secretion have been reported in babies. This results in severely low blood glucose which cannot be controlled without administering glucagon.
Glucagon can be given by injection to restore blood glucose lowered by insulin . It can increase glucose release from glycogen stores more than insulin can suppress it. The effect of glucagon is limited, so it is very important to eat a carbohydrate meal once the person has recovered enough to eat safely.
Mode Of Action And Effect On Metabolism
The effects of insulin are mediated by binding to its specific tyrosine kinase transmembrane receptor. The insulin receptor is a tetrameric protein with two extracellular -subunits and two intracellular -subunits. IR is present in both peripheral and central tissues. In peripheral tissues, insulin mediates metabolism via downstream activation of phosphatodylinostiol 3 kinase and insulin receptor substrate . The IRS-P13K pathway results in activation of different kinases. Recent evidence suggests that insulins action on hepatocytes stimulates glycolysis and lipogenesis via the protein complex mTORC2. Insulin is also considered to reduce gluconeogenesis by downregulating the Forkhead box class O transcription factors. Further effects on metabolism also include glycogen and protein synthesis.
Also Check: Is Gluten Free Better For Diabetics
Regulation Of Blood Glucose
Regulation of glucose in the body is done autonomically and constantly throughout each minute of the day. Normal BG levels should be between 60 and 140 mg/dL in order to supply cells of the body with its required energy. Brain cells dont require insulin to drive glucose into neurons however, there must still be normal amounts available. Too little glucose, called hypoglycemia, starves cells, and too much glucose creates a sticky, paralyzing effect on cells. Euglycemia, or blood sugar within the normal range, is naturally ideal for the bodys functions. A delicate balance between hormones of the pancreas, intestines, brain, and even adrenals is required to maintain normal BG levels.
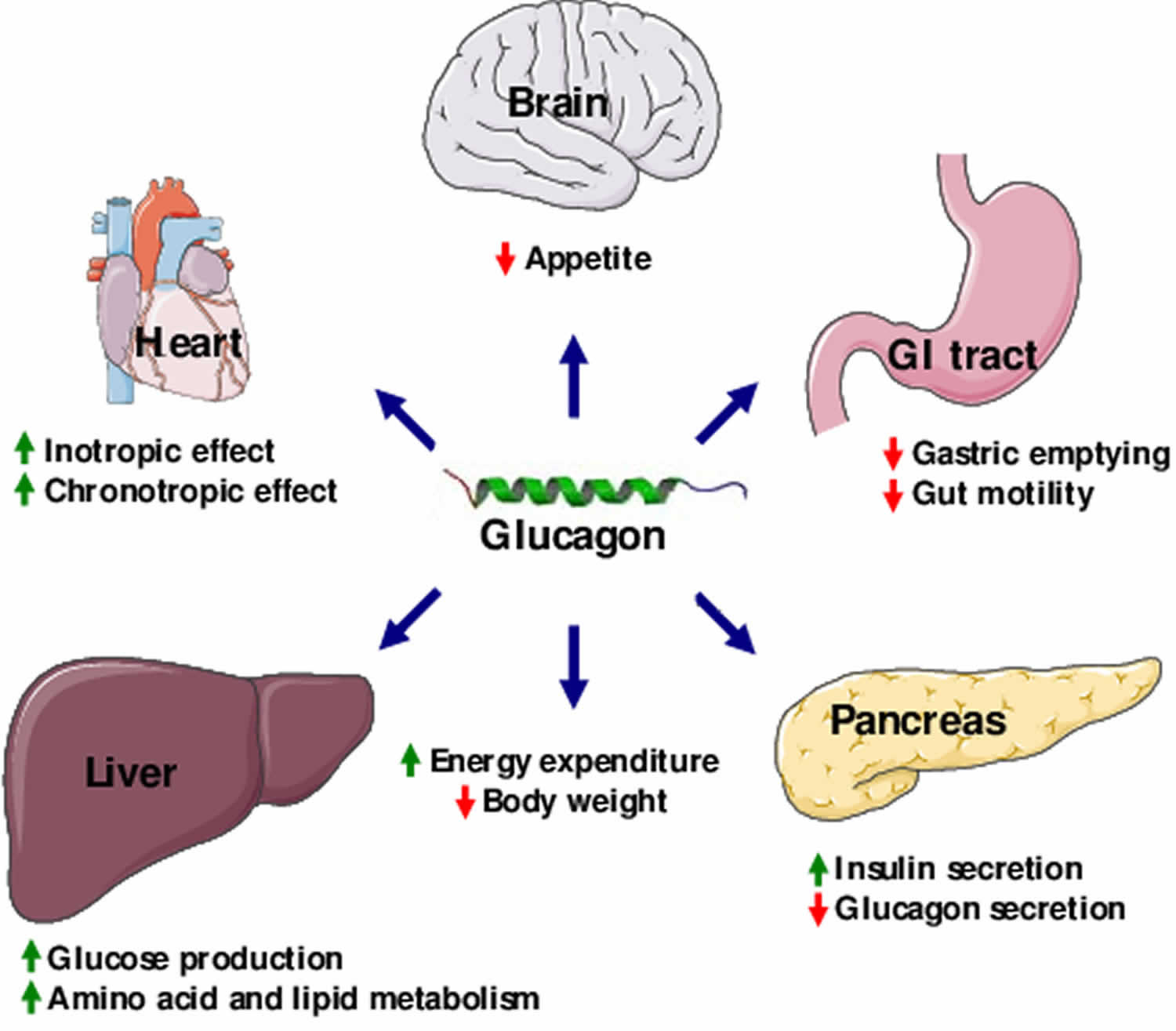
Glucagon Increases Energy Expenditure
In addition to a potential effect of glucagon on food intake, evidence suggests that glucagon contributes to a negative energy balance by stimulating energy expenditure. In humans, this effect has been observed in studies in which glucagon infusion resulted in increases in resting energy expenditure . However, the effect of endogenous glucagon on resting energy expenditure remains unclear. Also, the exact mechanisms behind the increase in resting energy expenditure elicited by exogenous glucagon remain to be determined. It has been speculated that glucagon activates brown adipose tissue , however this was recently challenged in an in vivo study that found no direct effect of glucagon on brown adipose tissue . Rodent studies indicate that the actions of glucagon to increase energy expenditure might be indirectly mediated partly by fibroblast growth factor 21 as glucagon-induced increase in energy expenditure is abolished in animals with FGF21 receptor deletion .
Recommended Reading: Arginine Insulin
Hepatic Glucose: Role Of The Liver In Diabetes
- 29shares
For a normal individual, low blood sugar is uncomfortable. But it is not dangerous and termed as Hunger. In a diabetic individual, the same feeling can be threatening. Our body maintains a blood-sugar level within the desired range throughout the day.
Insulin secretion, Insulin resistance, and hepatic glucose production are the 3 basic core defects of diabetes. For a diabetic individual, understanding these concepts is very important to monitor glucose levels. Hepatic glucose production is the formation of glucose in liver cells. It is regulated by hormones Insulin and Glucagon. In todays write up, we will overview the role of the Liver in Glucose Homeostasis.
Contents
Plasma Glucagon Responses To Hypoglycemia In Non
The median increases in plasma glucagon in response to hypoglycemia in nondiabetic and T1D subjects are shown in , and individual responses are shown in Supplementary Table 1. As shown in , the glucagon response to hypoglycemia was significantly reduced in the children and adolescents with T1D compared with that in the nondiabetic young-adult subjects . Moreover, 7 of the 21 subjects with T1D failed to achieve a glucagon response 12 pg/mL, and 4 of those subjects had absent responses . In contrast, only 1 of the 12 young-adult control subjects failed to achieve a glucagon response 12 pg/mL, and the rise in glucagon level was 11 pg/mL. The median incremental glucagon AUC was 1.5-fold higher in the nondiabetic subjects compared with the T1D subjects .
The difference in the glucagon response was noted despite achievement of similar nadir glucose levels in the two groups . Supplementary Fig. 2 depicts the change in the glucose and glucagon responses in the nondiabetic and T1D subjects during the hypoglycemic portion of the clamp.
Change in glucagon vs. duration of T1D .
Don’t Miss: Metformin With Or Without Food
Type 2 Diabetes: Too Much Glucagon
- Date:
- Uppsala University
- Summary:
- Patients with type 2 diabetes secrete not only too little insulin but also too much glucagon, which contributes to poor blood glucose control.
Patients with type 2 diabetes secrete not only too little insulin but also too much glucagon, which contributes to poor blood glucose control. A new study from Uppsala University suggests that this is because the glucagon-secreting -cells have become resistant to insulin.
In healthy individuals, insulin signals the body to absorb glucose, thereby reducing the sugar in the blood and providing energy to tissues. In patients with type 2 diabetes this mechanism fails, because the glucose-absorbing tissues become resistant to insulin and because too little of the hormone is released into the blood. This leads to elevated blood glucose and long-term complications that often become disabling or even life-threatening.
As expected, the experiments showed that glucagon is secreted during periods of low glucose, while high levels of the sugar efficiently block its release. However, in -cells of type 2 diabetics this regulation was disturbed and high glucose no longer blocked the release of glucagon. To find out why, Hmeadi and colleagues isolated the -cells and separated them from their tissue context in the pancreas. Surprisingly, the cells now behaved in a ‘diabetic’ manner and continued to secrete glucagon even when glucose was elevated.
Story Source:
Understanding The Processes Behind The Regulation Of Blood Glucose
20 April, 2004
VOL: 100, ISSUE: 16, PAGE NO: 56
Pat James, PhD, is senior lecturer in applied physiology
Roger McFadden, MSc, is senior lecturer in applied physiology both at the School of Health and Policy Studies, The University of Central England in Birmingham
Pat James, PhD, is senior lecturer in applied physiology
Glucose is one of the bodys principal fuels. It is an energy-rich monosaccharide sugar that is broken down in our cells to produce adenosine triphosphate. ATP is a small packet of chemical energy that powers the millions of biochemical reactions that take place in the body every second.
We obtain glucose from the food that we eat, predominantly starch-rich foods such as potatoes, rice, bread, and pasta. Starch is a polysaccharide that is broken down by digestive enzymes into individual glucose molecules.
In the small intestine, glucose is absorbed into the blood and travels to the liver via the hepatic portal vein. The hepatocytes absorb much of the glucose and convert it into glycogen, an insoluble polymer of glucose.
This is stored in the liver and can be reconverted into glucose when blood-glucose levels fall. Other types of simple sugars in our diet such as fructose, sucrose and lactose are also fuels that contribute to the production of ATP.
If glucose levels fall to too low a concentration or rise too high then this situation can lead to the neurological processes in the brain being compromised.
You May Like: Glucagon Secretion

