Paracrine Regulation Of Glucagon Secretion Via Camp
Several different mechanisms have been proposed for paracrine inhibition of glucagon secretion by increasing concentrations of glucose, and some of these involve changes in -cell cAMP. Somatostatin from -cells is an obvious candidate, as well as insulin and in the case of human islets also serotonin from -cells. It is evident that such mechanisms may help to inhibit glucagon release under hyperglycaemic conditions that stimulate secretion from both – and -cells. However, the significance of a paracrine mechanism for the physiologically important regulation of glucagon release in hypoglycaemia is less obvious. Glucagon secretion is thus regulated by lower glucose concentrations than those stimulating insulin release, and studies in different laboratories have indicated that glucose inhibition of glucagon secretion persists after interfering with somatostatin signalling and even in islets from somatostatin knockout mice.,
Beta Cells In Type 1 Diabetes
In type 1 diabetes, beta cells die from a misguided attack by the bodys immune system How and why that happens is not clear, but the results of a study published in early 2011 suggest that these pancreatic cells become stressed at the earliest stages of the disease process.
In mice, beta cells respond to this stress by triggering a cell death pathway that results in the loss of beta cell function, and ultimately the loss of beta cell mass.
The study authors from the Indiana University School of Medicine said the exciting findings raise the possibility that beta cell stress could be part of the trigger for the autoimmune process that leads to type 1 diabetes.
How Glucose Levels Are Regulated In The Blood Stream
Insulin and glucagon are like our yin and yang in regards to blood sugar levels. Both are produced by the pancreatic islets, the endocrine portion of the pancreas.
- Insulin is made by the beta cells in the pancreatic islets.
- Glucagon is made by the alpha cells in the pancreatic islets.
- Mnemonic: I remember this by remembering a vowel goes with a consonant, so Insulin starts with a vowel and is matched up with Beta cells, which starts with a consonant . Glucagon starts with a consonant and matches up with Alpha, which starts with a vowel .
Insulin and glucagon are both protein hormones which means if you had to give either one to a patient it would have to be done through an injection, as opposed to steroid hormones which are lipids and can be taken orally.
Insulin causes sugars in the blood stream to be transported into the cells, decreasing the blood sugar level. This sugar is usually used for creating ATP. In the liver, however, the glucose molecules join together to form a polysaccharide called glycogen. This process is called glycogenesis which literally means to produce glycogen.
Glucagon causes the breakdown of glycogen from the liver to release glucose into the blood stream, thus raising blood sugar levels. Interestingly enough, muscle glycogen can only be used by the muscle while liver glycogen can be re-released into the blood stream to be used by the muscles as well. This process is called glycogenolysis which literally means the breakdown of glycogen.
Recommended Reading: How Are Glucagon And Insulin Secretion Controlled
Physiological Role Of Insulin
Insulin is the pivotal hormone regulating cellular energy supply and macronutrient balance, directing anabolic processes of the fed state. Insulin is essential for the intra-cellular transport of glucose into insulin-dependent tissues such as muscle and adipose tissue. Signalling abundance of exogenous energy, adipose tissue fat breakdown is suppressed and its synthesis promoted. In muscle cells, glucose entry enables glycogen to be synthesised and stored, and for carbohydrates, rather than fatty acids to be utilised as the immediately available energy source for muscle contraction. Insulin therefore promotes glycogen and lipid synthesis in muscle cells, while suppressing lipolysis and gluconeogenesis from muscle amino acids. In the presence of an adequate supply of amino acids, insulin is anabolic in muscle.
When The Blood Glucose Level Goes Up
- Blood sugar rises
- The pancreas detects the rise
- The pancreas pumps out insulin into the blood
- Insulin helps the uptake of glucose into muscles and other cells
- This causes the blood glucose level to fall to its normal set point and
- The pancreas detects the fall and switches off insulin production.
Also Check: How To Keep Blood Sugar From Dropping
Synthesis And Release Of Insulin
Insulin is coded on the short arm of chromosome 11 and synthesised in the cells of the pancreatic islets of Langherhans as its precursor, proinsulin. Proinsulin is synthesised in the ribosomes of the rough endoplasmic reticulum from mRNA as pre-proinsulin. Pre-proinsulin is formed by sequential synthesis of a signal peptide, the B chain, the connecting peptide and then the A chain comprising a single chain of 100 amino acids. Removal of the signal peptide forms proinsulin, which acquires its characteristic 3 dimensional structure in the endoplasmic reticulum. Secretory vesicles transfer proinsulin from the RER to the Golgi apparatus, whose aqueous zinc and calcium rich environment favours formation of soluble zinc-containing proinsulin hexamers. As immature storage vesicles form from the Golgi, enzymes acting outside the Golgi convert proinsulin to insulin and C-peptide. Insulin forms zinc-containing hexamers which are insoluble, precipitating as chemically stable crystals at pH 5.5. When mature granules are secreted into the circulation by exocytosis, insulin, and an equimolar ratio of C-peptide are released. Proinsulin and zinc typically comprise no more than 6% of the islet cell secretion.
How Is Insulin Controlled
The main actions that insulin has are to allow glucose to enter cells to be used as energy and to maintain the amount of glucose found in the bloodstream within normal levels. The release of insulin is tightly regulated in healthy people in order to balance food intake and the metabolic needs of the body. This is a complex process and other hormones found in the gut and pancreas also contribute to this blood glucose regulation. When we eat food, glucose is absorbed from our gut into the bloodstream, raising blood glucose levels. This rise in blood glucose causes insulin to be released from the pancreas so glucose can move inside the cells and be used. As glucose moves inside the cells, the amount of glucose in the bloodstream returns to normal and insulin release slows down. Proteins in food and other hormones produced by the gut in response to food also stimulate insulin release. Hormones released in times of acute stress, such as adrenaline, stop the release of insulin, leading to higher blood glucose levels to help cope with the stressful event.
Insulin works in tandem with glucagon, another hormone produced by the pancreas. While insulin’s role is to lower blood sugar levels if needed, glucagon’s role is to raise blood sugar levels if they fall too low. Using this system, the body ensures that the blood glucose levels remain within set limits, which allows the body to function properly.
You May Like: How Many Points Does Metformin Lower Blood Sugar
Disorders Of The Endocrine System
Diabetes Mellitus
Dysfunction of insulin production and secretion, as well as the target cells responsiveness to insulin, can lead to a condition called diabetes mellitus. An increasingly common disease, diabetes mellitus has been diagnosed in more than 18 million adults in the United States, and more than 200,000 children. It is estimated that up to 7 million more adults have the condition but have not been diagnosed. In addition, approximately 79 million people in the US are estimated to have pre-diabetes, a condition in which blood glucose levels are abnormally high, but not yet high enough to be classified as diabetes.
There are two main forms of diabetes mellitus. Type 1 diabetes is an autoimmune disease affecting the beta cells of the pancreas. Certain genes are recognized to increase susceptibility. The beta cells of people with type 1 diabetes do not produce insulin thus, synthetic insulin must be administered by injection or infusion. This form of diabetes accounts for less than five percent of all diabetes cases.
Watch the video to view an animation describing the role of insulin and the pancreas in diabetes.
The Pancreas Is An Exocrine And Endocrine Organ
Anatomical organization of the pancreas. The exocrine function of the pancreas is mediated by acinar cells that secrete digestive enzymes into the upper small intestine via the pancreatic duct. Its endocrine function involves the secretion of various hormones from different cell types within the pancreatic islets of Langerhans. The micrograph shows the pancreatic islets. LM × 760 . Adapted from Human Anatomy and Physiology, an OpenStax College resource.
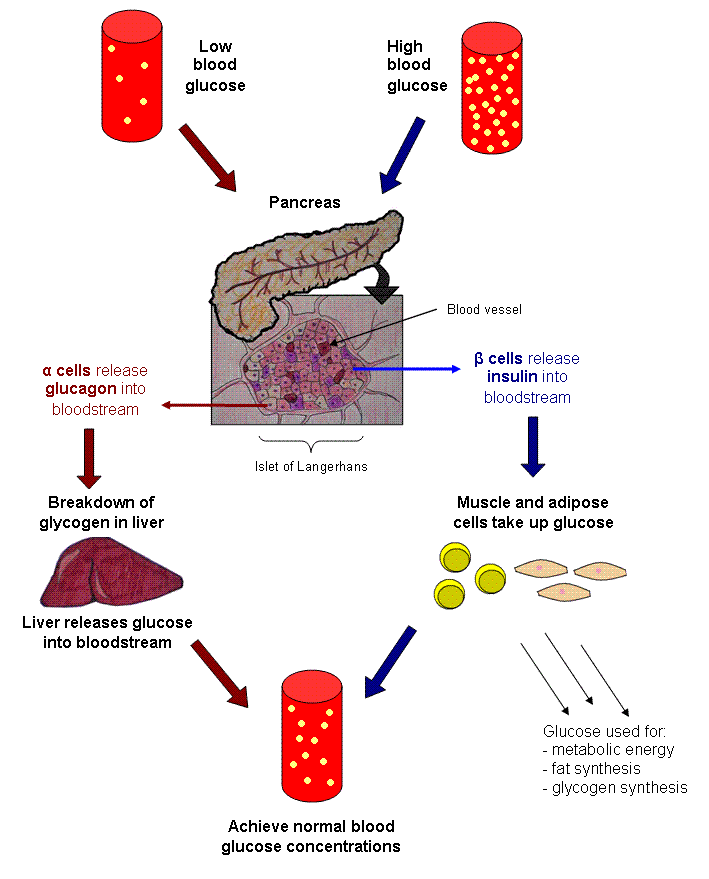
Maintenance of blood glucose levels by glucagon and insulin. When blood glucose levels are low, the pancreas secretes glucagon, which increases endogenous blood glucose levels through glycogenolysis. After a meal, when exogenous blood glucose levels are high, insulin is released to trigger glucose uptake into insulin-dependent muscle and adipose tissues as well as to promote glycogenesis.
You May Like: Insulin To Glucagon Ratio
Sites Of Insulin Action And Manifestations Of Insulin Resistance
The effects of insulin, insulin deficiency and insulin resistance vary according to the physiological function of the tissues and organs concerned, and their dependence on insulin for metabolic processes. Those tissues defined as insulin dependent, based on intracellular glucose transport, are principally adipose tissue and muscle. However, insulins actions are pleotropic and widespread, as are the manifestations of insulin resistance and the associated compensatory hyperinsulinaemia.
Regulation Of Blood Glucose
Regulation of glucose in the body is done autonomically and constantly throughout each minute of the day. Normal BG levels should be between 60 and 140 mg/dL in order to supply cells of the body with its required energy. Brain cells dont require insulin to drive glucose into neurons however, there must still be normal amounts available. Too little glucose, called hypoglycemia, starves cells, and too much glucose creates a sticky, paralyzing effect on cells. Euglycemia, or blood sugar within the normal range, is naturally ideal for the bodys functions. A delicate balance between hormones of the pancreas, intestines, brain, and even adrenals is required to maintain normal BG levels.
Also Check: At What A1c To Start Metformin
What Causes Release Of Insulin
causes insulinreleasedInsulin
Insulin is released from the beta cells in your pancreas in response to rising glucose in your bloodstream. After you eat a meal, any carbohydrates you’ve eaten are broken down into glucose and passed into the bloodstream. The pancreas detects this rise in blood glucose and starts to secrete insulin.
One may also ask, what hormone stimulates insulin release? Insulin secretion by the cells of the islets of Langerhans is primarily regulated by the d-glucose level in the extracellular fluid bathing the cells. Glucagon increases and somatostatin decreases insulin release via paracrine actions. Insulin release is stimulated by GH, cortisol, PRL, and the gonadal steroids.
Similarly one may ask, what inhibits the release of insulin?
Release of insulin is strongly inhibited by norepinephrine , which leads to increased blood glucose levels during stress.
What foods trigger insulin release?
The following can cause blood sugar and insulin levels to spike:
- sugary drinks, such as soda, juices, and sports drinks.
- processed foods and baked goods, which often contain trans fats.
- white rice, bread, and pasta.
- breakfast cereals with added sugar.
- yogurts with added sugar.
Energy Creation And Distribution
The function of insulin is to help transform glucose into energy and distribute it throughout your body, including the central nervous system and cardiovascular system.
Without insulin, cells are starved for energy and must seek an alternative source. This can lead to life threatening complications.
You May Like: Polygenic Hypercholesterolemia
Talk With Your Doctor
Knowing how your body works can help you stay healthy. Insulin and glucagon are two critical hormones your body makes to keep your blood sugar levels balanced. Its helpful to understand how these hormones function so you can work to avoid diabetes.
If you have more questions about insulin, glucagon, and blood glucose, talk to your doctor. Questions you have might include:
- Is my blood glucose at a safe level?
- Do I have prediabetes?
Insulin Is Continuously Released Into The Blood Stream
Insulin levels are carefully calibrated to keep the blood glucose just right.
Insulin is the main regulator of sugar in the bloodstream.
This hormone is made by beta cells and continuously released into the blood stream. Beta cells are found in the pancreas, which is an organ behind the stomach. Insulin levels in the blood stream are carefully calibrated to keep the blood glucose just right.
High insulin levels drive sugar out of the bloodstream into muscle, fat and liver cells where it is stored for future use. Low insulin levels allow sugar and other fuels to be released back into the blood stream.
Overnight and between meals, insulin levels in the blood stream are low and relatively constant. These low levels of insulin allow the body to tap into its stored energy sources and also to release sugar and other fuels from the liver. This overnight and between-meal insulin is referred to as background or basal insulin. When you havent eaten for a while, your blood sugar level will be somewhere between 60 to 100 mg/dl.
When eating, the amount of insulin released from the pancreas rapidly spikes. This burst of insulin that accompanies eating is called bolus insulin. After a meal, blood sugar levels peak at less than 140 mg/dl and then fall back to the baseline range. The high levels of insulin help the sugar get out of the blood stream and be stored for future use.
Recommended Reading: Metformin Side Effects In Males
V Insulin Secretory Pathway
The pancreatic b-cells act as a self-contained system to secrete insulin in response to changes in ambient blood glucose concentration, in order to maintain glucose homeostasis. Glucose is freely taken up into the b-cell via GLUT transporters, metabolized to produce ATP, which triggers a cascade of signals within the b cell necessary for glucose-induced insulin secretion. While GLUT2 has been traditionally assumed as the major mediator of glucose uptake into b-cells based on extrapolation from rodent studies and subsequent confirmation of GLUT2 transporters on human -cells , more recent studies in human islets suggest that the other insulin-independent glucose transporters GLUT1 and GLUT3 play a more important role, and are the main glucose transporters in human islet -cells . This redundancy explains why individuals with variants in the gene encoding GLUT2 do not have significant abnormalities in insulin secretion .
Figure 4. Diagrammatic illustration of insulin secretion. A low background secretion exists upon which is superimposed insulin secretory bursts stimulated by food intake. ).
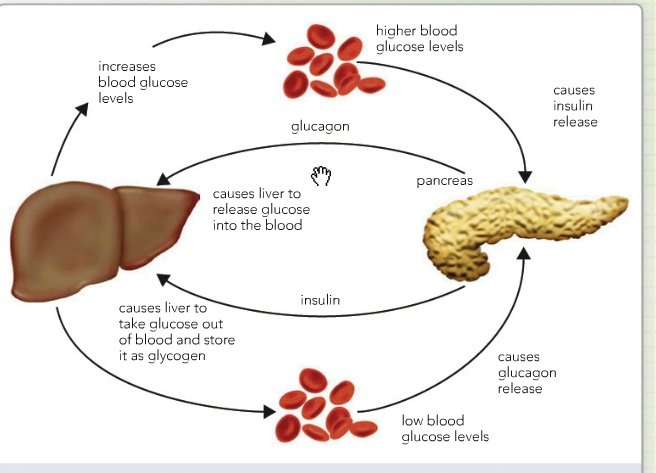
The Important Roles Of Insulin And Glucagon: Diabetes And Hypoglycemia
The human body wants blood glucose maintained in a very narrow range. Insulin and glucagon are the hormones which make this happen. Both insulin and glucagon are secreted from the pancreas, and thus are referred to as pancreatic endocrine hormones. The picture on the left shows the intimate relationship both insulin and glucagon have to each other. Note that the pancreas serves as the central player in this scheme. It is the production of insulin and glucagon by the pancreas which ultimately determines if a patient has diabetes, hypoglycemia, or some other sugar problem.
You May Like: Does Metformin Cause Anxiety
References And Recommended Reading
Ahrén, B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nature Reviews Drug Discovery8, 369385 . doi:10.1038/nrd2782.
Kebede, M. A., Alquier, T., et al. Lipid receptors and islet function: therapeutic implications? Diabetes, Obesity and Metabolism11, 1020 . doi: 10.1111/j.1463-1326.2009.01114.x.
Kim, H., Toyofuku, Y., et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nature Medicine16, 804808 . doi:10.1038/nm.2173.
Langenberg, C., Pascoe, L., et al. Common genetic variation in the melatonin receptor 1B gene is associated with decreased early-phase insulin response. Diabetologia52, 15371542 . doi: 10.1007/s00125-009-1392-x.
Regard, J. B., Kataoka, H., et al. Probing cell type-specific functions of G in vivo identifies GPCR regulators of insulin secretion. Journal of Clinical Investigation117, 40344043 . doi:10.1172/JCI32994.
Rosengren, A. H., Jokubka, R., et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science327, 217220 . doi: 10.1126/science.1176827.
Winzell, M. S. & Ahren, B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacology and Theraputics116, 437448 . doi:10.1016/j.pharmthera.2007.08.002.
Camp Effects On Secretory Granules And Exocytosis
Although the cAMP effects on electrical activity and i signalling contribute to the amplification of insulin secretion, the quantitatively most important effects are exerted on secretory granule trafficking and exocytosis. Both PKA and Epac promote secretory granule mobility, which leads to accelerated replenishment of readily releasable granules at the plasma membrane., , Before the vesicles are ready to fuse with the plasma membrane they undergo priming by yet incompletely understood mechanisms. For example, the Rab3-binding protein Rim2, which interacts with Epac2, has been implicated in granule priming. Granular acidification by the electrogenic vacuolar type H+-ATPase is one of the critical steps and simultaneous uptake of Cl and glutamate is believed to increase the proton gradient by neutralizing the charge. Indeed, PKA was recently found to stimulate vesicular uptake of glutamate. Previous studies have shown that SUR1 knockout mice lack the PKA-independent component of cAMP-amplified exocytosis, and this was ascribed to the loss of interaction between Epac2 and the SUR1 protein supposedly involved in granular Cl uptake. More recent studies indicate that the cAMP-regulated CFTR protein, in addition to regulating -cell electrical activity, is crucial for cAMP-amplified insulin secretion by stimulating vesicle priming via the Cl channel anoctamin 1.
Don’t Miss: Can Type 2 Diabetics Eat Bananas
Camp Effects On Metabolism
It has been observed that elevations of cAMP by GLP-1 and forskolin stimulate ATP production in mouse islets. In contrast, no effects on ATP production or oxygen consumption were observed in another study using conventional biochemical approaches. Whether cAMP influences metabolism thus remains controversial, and it is unclear how quantitatively important such an effect would be in relation to established actions on the more distal steps of the stimulus-secretion-coupling process.

